Tasty THC: Promises and Challenges of Cannabis Edibles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Selling Cannabis Regulation: Learning from Ballot Initiatives in the United States in 2012
ISSN 2054-1910 Selling cannabis regulation: Learning From Ballot Initiatives in the United States in 2012 Emily Crick*, Mark Cooke¥ and Dave Bewley-Taylorp Policy Brief 6 | November 2014 Key Points • In November 2012, Washington, Colorado, and Oregon voted on ballot initiatives to establish legally regulated markets for the production, sale, use and taxation of cannabis.1 Washington and Colorado’s measures won by wide margins, while Oregon’s lost soundly. • A majority of voters view cannabis in a negative light, but also feel that prohibition for non-medical and non-scientific purposes is not working. As a result, they are more likely to support well-crafted reform policies that include strong regulations and direct tax revenue to worthy causes such as public health and education. • Ballot measures are not the ideal method for passing complicated pieces of legislation, but sometimes they are necessary for controversial issues. Other states often follow in their footsteps, including via the legislature. • The successful campaigns in Washington and Colorado relied on poll-driven messaging, were well organised, and had significant financing. The Oregon campaign lacked these elements. • The Washington and Colorado campaigns targeted key demographic groups, particularly 30-50 year old women, who were likely to be initially supportive of reform but then switch their allegiance to the ‘no’ vote. • Two key messages in Washington and Colorado were that legalisation, taxation and regulation will (i) free up scarce law enforcement resources to focus on more serious crimes and (ii) will create new tax revenue for worthy causes. • National attitudes on legalising cannabis are changing, with more and more people supporting reform. -

The Legalization of Marijuana in Colorado: the Impact Vol
The Legalization of Marijuana in Colorado: The Impact Vol. 4/September 2016 PREPARED BY: ROCKY MOUNTAIN HIDTA INVESTIGATIVE SUPPORT CENTER STRATEGIC INTELLIGENCE UNIT INTELLIGENCE ANALYST KEVIN WONG INTELLIGENCE ANALYST CHELSEY CLARKE INTELLIGENCE ANALYST T. GRADY HARLOW The Legalization of Marijuana in Colorado: The Impact Vol. 4/September 2016 Table of Contents Acknowledgements Executive Summary ............................................................................................ 1 Purpose ..................................................................................................................................1 State of Washington Data ...................................................................................................5 Introduction .......................................................................................................... 7 Purpose ..................................................................................................................................7 The Debate ............................................................................................................................7 Background ...........................................................................................................................8 Preface ....................................................................................................................................8 Colorado’s History with Marijuana Legalization ...........................................................9 Medical Marijuana -

Cannabis Pest Management - a Perspective from Colorado Cultivated Cannabis Involves the Use of Two Species (Subspecies?) That Freely Interbreed
Cannabis Pest Management - A Perspective from Colorado Cultivated Cannabis involves the use of two species (subspecies?) that freely interbreed Cannabis indica Cannabis sativa What type of crop is cannabis? Types of Cannabis Crops • Medical/Recreational Use –Marijuana • CBD (cannabidiol) Production –Non-psychoactive extracts • Hemp grown for seed, fiber Present Status of State Laws Regarding Legality of Medical and/or Recreational Marijuana Key Colorado State Laws Regarding Cannabis • November 2000 – Passage of Amendment 20 – Allows usage of Cannabis for patients with written medical permission (“medical marijuana”) – Patients may grow up to 6 plants – Patients may acquire Cannabis from a caregiver or from non-state affiliated clubs/organizations (dispensaries) Some Background – Key Date • November 2012 – Passage of Amendment 64 – Allows personal use of Cannabis for all uses (e.g., recreational use) – Establishes regulations on production and sale of Cannabis – Directed that a system be established to allow hemp production within the state Marijuana Production • Involves C. sativa, C. indica and hybrids • Primary compound THC – Secondary cannabinoids often important • End uses – Whole buds (inhaled) – Extracts • Edibles • Inhalation (vaping) • Salves, ointments 10 mg THC is standardized serving size Each plant is tagged and tracked through the entire production stage – through end point distribution. The crop is clonally propagated – all female plants. Culture is with drip irrigation into pots or through hydroponic production Medical/Recreational -

DRONABINOL What Should You Do If You FORGET a What Other PRECAUTIONS Should Dose? You Follow While Using This Drug?
DRONABINOL What should you do if you FORGET a What other PRECAUTIONS should dose? you follow while using this drug? Other NAMES : Marinol , delta – 9 - If you miss a dose of dronabinol, take it Before starting dronabinol, inform your tetrahydrocannabinol as soon as possible. However, if it is doctor if you have already had in the time for your next dose, do not double past adverse reactions to dronabinol, WHY is this drug prescribed? the dose, just carry on with your regular nabilone (Cesamet ) or marijuana schedule. (pot). Also, notify your doctor if you are Dronabinol is a narcotic drug used to allergic to sesame oil, if you have heart treat severe nausea and vomiting. It is What ADVERSE EFFECTS can this disease (hypertension, abnormal in a family of drugs called cannabinoids drug cause? What should you do heartbeat, angina), or a history of (eg. marijuana). It is also used to about them? emotional disorders such as depression, increase appetite and weight gain. bipolar disease (manic depression), and Dronabinol can cause unsteadiness, schizophrenia (psychosis, HOW should this drug be taken? dizziness, difficulty concentrating, hallucinations). drowsiness, euphoria, abnormal Dronabinol is available in 2.5 mg, 5 mg thinking, fatigue, anxiety, confusion, As this drug may cause some people to and 10 mg capsules. To increase hallucinations, and paranoia. become dizzy, drowsy or less alert than appetite and body weight, the usual Nausea, vomiting, abdominal pain, and normal, you should NOT drive, use initial dose is 2.5 mg twice daily. facial redness may also occur. machinery or do anything else that Depending on the results obtained, your could be dangerous if you are dizzy or doctor might decide to slowly increase These effects may go away during are not alert. -

DRONABINOL- Dronabinol Capsule Actavis Pharma, Inc
DRONABINOL- dronabinol capsule Actavis Pharma, Inc. ---------- DRONABINOL Capsules, USP 2.5 mg, 5 mg, 10 mg CIII Rx only DESCRIPTION Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9- trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in dronabinol capsules, USP, is synthetic delta-9-tetrahydrocannabinol (delta-9-THC). Delta-9-tetrahydrocannabinol is also a naturally occurring component of Cannabis sativa L. (Marijuana). Dronabinol is a light yellow resinous oil that is sticky at room temperature and hardens upon refrigeration. Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7. Capsules for oral administration: Dronabinol capsules are supplied as round, soft gelatin capsules containing either 2.5 mg, 5 mg, or 10 mg dronabinol. Each dronabinol capsule strength is formulated with the following inactive ingredients: 2.5 mg capsule contains gelatin, glycerin, sesame oil, and titanium dioxide; 5 mg capsule contains iron oxide red and iron oxide black, gelatin, glycerin, sesame oil, and titanium dioxide; 10 mg capsule contains iron oxide red and iron oxide yellow, gelatin, glycerin, sesame oil, and titanium dioxide. CLINICAL PHARMACOLOGY Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS), including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol and other cannabinoids. -

The Rise and Decline of Cannabis Prohibition the History of Cannabis in the UN Drug Control System and Options for Reform
TRANSNATIONAL I N S T I T U T E THE RISE AND DECLINE OF CANNABIS PROHIBITION THE HISTORY OF CANNABIS IN THE UN DruG CONTROL SYSTEM AND OPTIONS FOR REFORM 3 The Rise and Decline of Cannabis Prohibition Authors Dave Bewley-Taylor Tom Blickman Martin Jelsma Copy editor David Aronson Design Guido Jelsma www.guidojelsma.nl Photo credits Hash Marihuana & Hemp Museum, Amsterdam/ Barcelona Floris Leeuwenberg Pien Metaal UNOG Library/League of Nations Archives UN Photo Printing Jubels, Amsterdam Contact Transnational Institute (TNI) De Wittenstraat 25 1052 AK Amsterdam Netherlands Tel: +31-(0)20-6626608 Fax: +31-(0)20-6757176 [email protected] www.tni.org/drugs www.undrugcontrol.info www.druglawreform.info Global Drug Policy Observatory (GDPO) Research Institute for Arts and Humanities Rooms 201-202 James Callaghan Building Swansea University Financial contributions Singleton Park, Swansea SA2 8PP Tel: +44-(0)1792-604293 This report has been produced with the financial www.swansea.ac.uk/gdpo assistance of the Hash Marihuana & Hemp Museum, twitter: @gdpo_swan Amsterdam/Barcelona, the Open Society Foundations and the Drug Prevention and Information Programme This is an Open Access publication distributed under (DPIP) of the European Union. the terms of the Creative Commons Attribution License The contents of this publication are the sole responsibility (http://creativecommons.org/licenses/by/2.0), which of TNI and GDPO and can under no circumstances be permits unrestricted use, distribution, and reproduction regarded as reflecting the position of the donors. in any medium, provided the original work is properly cited. TNI would appreciate receiving a copy of the text in which this document is used or cited. -

Will Marijuana Legalization Increase Hospitalizations and Emergency Room Visits?
nabi Can s P y o l c i i c l y o P S e s r i i b e a s n n a C WILL MARIJUANA LEGALIZATION INCREASE HOSPITALIZATIONS AND EMERGENCY ROOM VISITS? By Allie Howell July 2018 Since marijuana legalization will likely increase the availability and convenience of consuming marijuana, there is concern that it will also increase health emergencies. An especially prominent concern is that children will be more likely to ingest marijuana in states that have legalized adult use. Reason Foundation WILL MARIJUANA LEGALIZATION INCREASE HOSPITALIZATIONS AND EMERGENCY ROOM VISITS? 2 AVAILABILITY OF EDIBLES MAY INCREASE HOSPITALIZATIONS Traditionally, adult hospitalizations from marijuana use were almost unheard of. Legalization, however, has increased the availability of marijuana products, especially edibles that contain multiple “doses” of delta-9-tetrahydrocannabinol (THC). Edibles have been cited as a common cause for marijuana emergencies because it takes longer to feel the effects of the drug, which may cause users to ingest more. By the time the peak effect of an edible is felt, the user may be extremely high and this may cause them to seek medical attention for acute intoxication.1 MARIJUANA-RELATED EMERGENCIES Edibles have also increased the prevalence of pediatric ingestion because of packaging that makes marijuana products look like candy or desserts. Between 2005 and 2011, there were 985 unintentional pediatric exposures (children nine and younger) in the U.S.2 In Colorado, emergency room visits for teenagers and young adults ages 13–21 increased from 1.8 per 1,000 in 2009 to 4.9 per 1,000 in 2015.3 Another study found that parents at an Aurora, Colorado children's hospital disclosed a history of marijuana exposure in 56% of patients (18 patients) in 2014 and 2015 compared with 19% of patients (three patients) in 2012 and 2013. -
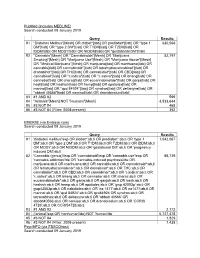
Diabetes Mellitus
PubMed (includes MEDLINE) Search conducted 09 January 2019 Query Results #1 "Diabetes Mellitus"[Mesh] OR diabet*[tiab] OR prediabet*[tiab] OR "type 1 630,566 DM"[tiab] OR "type 2 DM"[tiab] OR T1DM[tiab] OR T2DM[tiab] OR IDDM[tiab] OR MODY[tiab] OR NIDDM[tiab] OR "gestational DM"[tiab] #2 "Cannabis"[Mesh] OR "Cannabinoids"[Mesh] OR "Marijuana 52,197 Smoking"[Mesh] OR "Marijuana Use"[Mesh] OR "Marijuana Abuse"[Mesh] OR "Medical Marijuana"[Mesh] OR marijuana[tiab] OR marihuana[tiab] OR cannabis[tiab] OR cannabinoid*[tiab] OR tetrahydrocannabinol*[tiab] OR dronabinol*[tiab] OR THC[tiab] OR cannabidiol*[tiab] OR CBD[tiab] OR cannabinol*[tiab] OR "c.indica"[tiab] OR "c.sativa"[tiab] OR bhang[tiab] OR cannador[tiab] OR charas[tiab] OR eucannabinolide*[tiab] OR ganja[tiab] OR hash[tiab] OR hashish[tiab] OR hemp[tiab] OR epidiolex[tiab] OR marinol[tiab] OR "qcd 84924"[tiab] OR syndros[tiab] OR deltanyne[tiab] OR "abbott 40566"[tiab] OR namisol[tiab] OR dronabinolum[tiab] #3 #1 AND #2 599 #4 "Animals"[Mesh] NOT "Humans"[Mesh] 4,533,644 #5 #3 NOT #4 468 #6 #3 NOT #4 (Filter: 2008-present) 352 EMBASE (via Embase.com) Search conducted 09 January 2019 Query Results #1 'diabetes mellitus'/exp OR diabet*:ab,ti OR prediabet*:ab,ti OR 'type 1 1,042,067 DM':ab,ti OR 'type 2 DM':ab,ti OR T1DM:ab,ti OR T2DM:ab,ti OR IDDM:ab,ti OR MODY:ab,ti OR NIDDM:ab,ti OR 'gestational DM':ab,ti OR 'pregnancy induced DM':ab,ti #2 'Cannabis (genus)'/exp OR 'cannabinoid'/exp OR 'cannabis use'/exp OR 88,739 'cannabis addiction'/de OR 'cannabis-induced psychosis'/de OR marijuana:ab,ti -

The Board of Oregon National Organization for the Reform Of
This letter is on behalf of the entire Oregon NORML board, including me. --- We the board of Oregon NORML oppose Senate Bill 218 and are asking our House of Representatives to reject this bill outright. S.B. 218 accelerates the conditions that allow predatory international investors from dominating our artificially contained in-state market. The West Coast, and particularly Oregon, has supplied the nation cannabis for decades before legalization. The oversupply of cannabis should not be addressed through license caps, but rather a rigorous defense of our legacy industry by our state representatives and leaders, particularly Governor Brown. Governor Brown should enter into negotiations immediately with the federal government for the ability to begin exporting Oregon’s legal tested oversupply to other states. Currently there are patients in places like Utah, Louisiana and Arkansas who are using untested illegal cannabis while they wait for reasonable and responsibly regulated systems like Oregon’s to supply medical demand. Nevada, a legal state that we share a border with, has high prices and an undersupply. A capping of licenses will make it even more difficult for women and minority groups to open businesses in their communities. Oregon should be working to improve diversity in the industry, not limit it. Crucially, with the rise of highly-taxed legal cannabis in Oregon -- which has brought in $173 million in tax revenues since 2016 -- vulnerable cannabis patients in your communities have completely fallen by the wayside. Patients with qualifying conditions written into Oregon’s state law have lost access to caregivers and as a result, patient numbers are dwindling. -

Environmental Impacts of Cannabis in Colorado
Environmental Impacts of Cannabis in Colorado Presented by Kaitlin Urso 5/19/2020 Environmental Impacts of Cannabis Cultivation Energy for lighting and HVAC ◦ NOx and other emissions from generators in rural areas & fuel burning Water treatment, irrigation, run-off, erosion, and wastewater effluent Plant waste, universal waste, and hazardous waste Air emissions Terpenes= VOCs Chemical use – pesticides, fertilizers, and solvents Environmental Impacts of Product Processing Solvent evaporation contributes to ozone formation (VOCs) CO2 releases Cleaning products Haz Waste from solvent extraction Plant waste NEW National Cannabis Industry to release Environmental Sustainability White Paper – June 2020 Environmental Impacts Best Management Practices Policies Necessary to position the cannabis industry as a leader in environmental sustainability and to help influence environmental policy that comes along with federal legalization. Project Managed by Kaitlin Urso, CO SBEAP As cannabis grows, it naturally emits odorous terpenes. Terpenes are volatile organic compounds. *Terpenes VOC (odor) VOC VOC VOC VOC VOC chemically react with NOx in sunlight to form ground-level ozone. *Terpenes (odor) Ozone VOC VOC NOx NOx VOC VOC VOC NO x NOx NOx VOC emissions from cannabis cultivation are subject to state air quality regulations . Marijuana cultivation is considered an “agricultural activity” ‒ Even when it is grown indoors . Agricultural activities are exempt (for the most part) from state air quality regulations ‒ CRS 25-7- 109(8)(a) 7 Solvents Evaporate VOCs during Extraction and Cleaning- Compliance Point VOC Common Solvents VOC ◦ Propane VOC VOC VOC ◦ Butane VOC VOC ◦ Ethanol ◦ Isopropyl Alcohol Mass Balance for calculating air emissions ◦ Purchased minus inventory minus waste pick-up ◦ Assume everything else evaporates www.colorado.gov/cdphe/greencannabis/air-quality VOC emissions from extraction facilities ARE subject to air quality regulations! . -

A Proposal to Federally Legalize Recreational Cannabis
A PROPOSAL TO FEDERALLY LEGALIZE RECREATIONAL CANNABIS by Andrew J. Baldwin A capstone project submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Arts in Public Management Baltimore, Maryland May 2021 2021 Andrew Baldwin All Rights Reserved ABSTRACT This policy proposal examines the issue of the disparity between Federal and State positions regarding cannabis legalization with regards to State cross-border criminal activity and the preeminence of Federal law. The proposal is based on examination of the history of Federal cannabis prohibition policies, State legalization policies, cannabis’ part in drug law enforcement, and the growing legal cannabis industry. No Federal court has yet to tackle the disparity between Federal prohibition of cannabis and the 33 States that have legalized cannabis to some degree (either medicinally or recreationally). This demonstrates a reluctance to truly continue Federal prohibition out of nothing more than decades of flawed drug enforcement policies. Therefore, it was recommended that, in light of the U.S. House of Representatives passing the MORE Act of 2019, the Federal government should legalize cannabis products recreationally in order to more effectively establish a legal cannabis industry with regulations similar to the alcohol and tobacco industries currently. Capstone Advisor: Dr. Paul Weinstein ii Acknowledgements To my wonderful wife, Meagan, who has encouraged me through this rigorous program more times than I can count – thank you. Also, to -

Marijuana in Oregon: a Primer for Clinicians
Legalized Marijuana in Oregon: A Primer for Clinicians Katrina Hedberg, MD, MPH Health Officer & State Epidemiologist History • Originated Central Asia • 3000 BC: Chinese Emperor Shen Nung • Ancient Greeks and Romans familiar with cannabis • 1840’s: Dr. WB O’Shaughnessy, a surgeon working for the British East India Company • 1800’s: Hemp grown in US by President Washington • 1937: US marijuana tax law making it illegal Oregon Public Health Division 2 Marijuana: US Legal Status US DEA Controlled Substances Act Schedule 1 • High potential for abuse • No currently accepted medical use in US (federal level); clinical trials lacking 2018: State Laws • 30 States Medical MJ • 9 recreational Oregon Public Health Division 3 Oregon Medical Marijuana Act • December 1998: Oregon voters approved Ballot Measure 67 • Allows medical use of marijuana (for specific conditions) • Established a state-controlled permit system (the OMMP) • Protects patients and doctors from penalties Oregon Public Health Division 4 Marijuana Sales in Oregon • Jan 2014: Oregon Legislature approved Medical Marijuana Dispensaries (OHA regulates) • July 2015: recreational marijuana became legal • Oct 2015: early sales of retail marijuana allowed at medical dispensaries • Oct 2016: retail marijuana stores open (OLCC regulates) Oregon Public Health Division 5 Objectives • Be familiar with cannabis, cannabinoids, and medical conditions for which scientific evidence supports effectiveness. • Describe elements of Oregon’s clinical guidelines for physicians when recommending use of medical