Recreational Water Illnesses
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

BD-CS-057, REV 0 | AUGUST 2017 | Page 1
EXPLIFY RESPIRATORY PATHOGENS BY NEXT GENERATION SEQUENCING Limitations Negative results do not rule out viral, bacterial, or fungal infections. Targeted, PCR-based tests are generally more sensitive and are preferred when specific pathogens are suspected, especially for DNA viruses (Adenovirus, CMV, HHV6, HSV, and VZV), mycobacteria, and fungi. The analytical sensitivity of this test depends on the cellularity of the sample and the concentration of all microbes present. Analytical sensitivity is assessed using Internal Controls that are added to each sample. Sequencing data for Internal Controls is quantified. Samples with Internal Control values below the validated minimum may have reduced analytical sensitivity or contain inhibitors and are reported as ‘Reduced Analytical Sensitivity’. Additional respiratory pathogens to those reported cannot be excluded in samples with ‘Reduced Analytical Sensitivity’. Due to the complexity of next generation sequencing methodologies, there may be a risk of false-positive results. Contamination with organisms from the upper respiratory tract during specimen collection can also occur. The detection of viral, bacterial, and fungal nucleic acid does not imply organisms causing invasive infection. Results from this test need to be interpreted in conjunction with the clinical history, results of other laboratory tests, epidemiologic information, and other available data. Confirmation of positive results by an alternate method may be indicated in select cases. Validated Organisms BACTERIA Achromobacter -

List of the Pathogens Intended to Be Controlled Under Section 18 B.E
(Unofficial Translation) NOTIFICATION OF THE MINISTRY OF PUBLIC HEALTH RE: LIST OF THE PATHOGENS INTENDED TO BE CONTROLLED UNDER SECTION 18 B.E. 2561 (2018) By virtue of the provision pursuant to Section 5 paragraph one, Section 6 (1) and Section 18 of Pathogens and Animal Toxins Act, B.E. 2558 (2015), the Minister of Public Health, with the advice of the Pathogens and Animal Toxins Committee, has therefore issued this notification as follows: Clause 1 This notification is called “Notification of the Ministry of Public Health Re: list of the pathogens intended to be controlled under Section 18, B.E. 2561 (2018).” Clause 2 This Notification shall come into force as from the following date of its publication in the Government Gazette. Clause 3 The Notification of Ministry of Public Health Re: list of the pathogens intended to be controlled under Section 18, B.E. 2560 (2017) shall be cancelled. Clause 4 Define the pathogens codes and such codes shall have the following sequences: (1) English alphabets that used for indicating the type of pathogens are as follows: B stands for Bacteria F stands for fungus V stands for Virus P stands for Parasites T stands for Biological substances that are not Prion R stands for Prion (2) Pathogen risk group (3) Number indicating the sequence of each type of pathogens Clause 5 Pathogens intended to be controlled under Section 18, shall proceed as follows: (1) In the case of being the pathogens that are utilized and subjected to other law, such law shall be complied. (2) Apart from (1), the law on pathogens and animal toxin shall be complied. -

Genetic and Functional Studies of the Mip Protein of Legionella
t1.ì. Genetic and Functional Studies of the Mip Protein of Legionella Rodney Mark Ratcliff, BSc (Hons)' MASM Infectious Diseases Laboratories Institute of Medical and Veterinary Science and Department of Microbiology and Immunology UniversitY of Adelaide. Adelaide, South Australia A thesis submitted to the University of Adelaide for the degree of I)octor of Philosophy 15'h March 2000 amended 14th June 2000 Colonies of several Legionella strains on charcoal yeast extract agar (CYE) after 4 days incubation at 37"C in air. Various magnifications show typical ground-glass opalescent appearance. Some pure strains exhibit pleomorphic growth or colour. The top two photographs demonstrate typical red (LH) and blue-white (RH) fluorescence exhibited by some species when illuminated by a Woods (IJV) Lamp. * t Table of Contents .1 Chapter One: Introduction .1 Background .............'. .2 Morphology and TaxonomY J Legionellosis ............. 5 Mode of transmission "..'....'. 7 Environmental habitat 8 Interactions between Legionella and phagocytic hosts 9 Attachment 11 Engulfment and internalisation.'.. 13 Intracellular processing 13 Intracellular replication of legionellae .. " "' " "' 15 Host cell death and bacterial release 18 Virulence (the Genetic factors involved with intracellular multiplication and host cell killing .20 icm/dot system) Legiolysin .25 Msp (Znn* metaloprotease) ...'..... .25 .28 Lipopolysaccharide .29 The association of flagella with disease.. .30 Type IV fimbriae.... .31 Major outer membrane proteins....'.......'. JJ Heat shock proteins'.'. .34 Macrophage infectivity potentiator (Mip) protein Virulenceiraits of Legionella species other than L. pneumophila..........' .39 phylogeny .41 Chapter One (continued): Introduction to bacterial classification and .41 Identificati on of Legionella...'.,..'.. .46 Phylogeny .52 Methods of phylogenetic analysis' .53 Parsimony methods.'.. .55 Distance methods UPGMA cluster analYsis.'.'... -
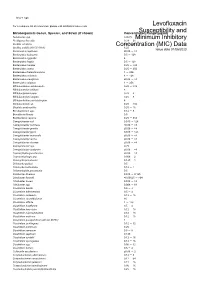
Susceptibility and Resistance Data
toku-e logo For a complete list of references, please visit antibiotics.toku-e.com Levofloxacin Microorganism Genus, Species, and Strain (if shown) Concentration Range (μg/ml)Susceptibility and Aeromonas spp. 0.0625 Minimum Inhibitory Alcaligenes faecalis 0.39 - 25 Bacillus circulans Concentration0.25 - 8 (MIC) Data Bacillus subtilis (ATCC 6051) 6.25 Issue date 01/06/2020 Bacteroides capillosus ≤0.06 - >8 Bacteroides distasonis 0.5 - 128 Bacteroides eggerthii 4 Bacteroides fragilis 0.5 - 128 Bacteroides merdae 0.25 - >32 Bacteroides ovatus 0.25 - 256 Bacteroides thetaiotaomicron 1 - 256 Bacteroides uniformis 4 - 128 Bacteroides ureolyticus ≤0.06 - >8 Bacteroides vulgatus 1 - 256 Bifidobacterium adolescentis 0.25 - >32 Bifidobacterium bifidum 8 Bifidobacterium breve 0.25 - 8 Bifidobacterium longum 0.25 - 8 Bifidobacterium pseudolongum 8 Bifidobacterium sp. 0.25 - >32 Bilophila wadsworthia 0.25 - 16 Brevibacterium spp. 0.12 - 8 Brucella melitensis 0.5 Burkholderia cepacia 0.25 - 512 Campylobacter coli 0.015 - 128 Campylobacter concisus ≤0.06 - >8 Campylobacter gracilis ≤0.06 - >8 Campylobacter jejuni 0.015 - 128 Campylobacter mucosalis ≤0.06 - >8 Campylobacter rectus ≤0.06 - >8 Campylobacter showae ≤0.06 - >8 Campylobacter spp. 0.25 Campylobacter sputorum ≤0.06 - >8 Capnocytophaga ochracea ≤0.06 - >8 Capnocytophaga spp. 0.006 - 2 Chlamydia pneumonia 0.125 - 1 Chlamydia psittaci 0.5 Chlamydia trachomatis 0.12 - 1 Chlamydophila pneumonia 0.5 Citrobacter diversus 0.015 - 0.125 Citrobacter freundii ≤0.00625 - >64 Citrobacter koseri 0.015 - -

Aquascreen® Legionella Species Qpcr Detection Kit
AquaScreen® Legionella species qPCR Detection Kit INSTRUCTIONS FOR USE FOR USE IN RESEARCH AND QUALITY CONTROL Symbols Lot No. Cat. No. Expiry date Storage temperature Number of reactions Manufacturer INDICATION The AquaScreen® Legionella species qPCR Detection kit is specifically designed for the quantitative detection of several Legionella species in water samples prepared with the AquaScreen® FastExt- ract kit. Its design complies with the requirements of AFNOR T90-471 and ISO/TS 12869:2012. Legionella are ubiquitous bacteria in surface water and moist soil, where they parasitize protozoa. The optimal growth temperature lies between +15 and +45 °C, whereas these gram-negative bacteria are dormant below 20 °C and do not survive above 60 °C. Importantly, Legionella are well-known as opportunistic intracellular human pathogens causing Legionnaires’ disease and Pontiac fever. The transmission occurs through inhalation of contami- nated aerosols generated by an infected source (e.g. human-made water systems like shower- heads, sink faucets, heaters, cooling towers, and many more). In order to efficiently prevent Legionella outbreaks, water safety control measures need syste- matic application but also reliable validation by fast Legionella testing. TEST PRINCIPLE The AquaScreen® Legionella species Kit uses qPCR for quantitative detection of legionella in wa- ter samples. In contrast to more time-consuming culture-based methods, AquaScreen® assays need less than six hours including sample preparation and qPCR to reliably detect Legionella. Moreover, the AquaScreen® qPCR assay has proven excellent performance in terms of specificity and sensitivity: other bacterial genera remain undetected whereas linear quantification is obtai- ned up to 1 x 106 particles per sample, therefore requiring no material dilution. -

Immunoproteomic Identification of Biomarkers for Diagnosis of Legionellosis
Immunoproteomic identification of biomarkers for diagnosis of legionellosis Submitted in total fulfilment of the requirements for the degree of Doctor of philosophy by Kaylass Poorun Department of Chemistry and Biotechnology Faculty of Science, Engineering and Technology Swinburne University of Technology Australia 2014 Abstract Abstract Legionellosis, a disease with significant mortality and morbidity rates, is considered to be the second most frequent cause of severe community-acquired pneumonia. It is difficult to distinguish from other types of pneumonia due to similar clinical manifestations. Several studies have demonstrated the inadequacies of current diagnostic tests for confirming Legionella infections. This study was aimed at identifying biomarkers that can be used in an improved test. A comparative proteomic analysis, using DIGE, was carried out between L. pneumophila ATCC33152 and L. longbeachae NSW150 and D4968 isolates. While many homologous proteins were found to be commonly expressed, numerous others were identified to be differentially expressed under similar in vitro conditions suggesting that the two species have different lifestyles and infection strategies. The bacterial immunoglobulin domain containing protein, found to share sequence homology to Type V secretion proteins intimin and invasin, is not known to be present in Legionella. Human sera containing antibodies against Legionella from a set of blind samples were identified by ELISA. Downstream analyses revealed that diverse immunogens may be responsible for eliciting immune response in different Legionella species which in turn show little to no congeneric cross-reactivity. To the best of our knowledge, this is a unique finding not previously reported. Several serological diagnostic tests currently in use do not include many Legionella species in their testing panel, which may be a reason for many Legionella species being under-reported. -

Microbial and Mineralogical Characterizations of Soils Collected from the Deep Biosphere of the Former Homestake Gold Mine, South Dakota
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln US Department of Energy Publications U.S. Department of Energy 2010 Microbial and Mineralogical Characterizations of Soils Collected from the Deep Biosphere of the Former Homestake Gold Mine, South Dakota Gurdeep Rastogi South Dakota School of Mines and Technology Shariff Osman Lawrence Berkeley National Laboratory Ravi K. Kukkadapu Pacific Northwest National Laboratory, [email protected] Mark Engelhard Pacific Northwest National Laboratory Parag A. Vaishampayan California Institute of Technology See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usdoepub Part of the Bioresource and Agricultural Engineering Commons Rastogi, Gurdeep; Osman, Shariff; Kukkadapu, Ravi K.; Engelhard, Mark; Vaishampayan, Parag A.; Andersen, Gary L.; and Sani, Rajesh K., "Microbial and Mineralogical Characterizations of Soils Collected from the Deep Biosphere of the Former Homestake Gold Mine, South Dakota" (2010). US Department of Energy Publications. 170. https://digitalcommons.unl.edu/usdoepub/170 This Article is brought to you for free and open access by the U.S. Department of Energy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in US Department of Energy Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Gurdeep Rastogi, Shariff Osman, Ravi K. Kukkadapu, Mark Engelhard, Parag A. Vaishampayan, Gary L. Andersen, and Rajesh K. Sani This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ usdoepub/170 Microb Ecol (2010) 60:539–550 DOI 10.1007/s00248-010-9657-y SOIL MICROBIOLOGY Microbial and Mineralogical Characterizations of Soils Collected from the Deep Biosphere of the Former Homestake Gold Mine, South Dakota Gurdeep Rastogi & Shariff Osman & Ravi Kukkadapu & Mark Engelhard & Parag A. -

The Role of Lipids in Legionella-Host Interaction
International Journal of Molecular Sciences Review The Role of Lipids in Legionella-Host Interaction Bozena Kowalczyk, Elzbieta Chmiel and Marta Palusinska-Szysz * Department of Genetics and Microbiology, Institute of Biological Sciences, Faculty of Biology and Biotechnology, Maria Curie-Sklodowska University, Akademicka St. 19, 20-033 Lublin, Poland; [email protected] (B.K.); [email protected] (E.C.) * Correspondence: [email protected] Abstract: Legionella are Gram-stain-negative rods associated with water environments: either nat- ural or man-made systems. The inhalation of aerosols containing Legionella bacteria leads to the development of a severe pneumonia termed Legionnaires’ disease. To establish an infection, these bacteria adapt to growth in the hostile environment of the host through the unusual structures of macromolecules that build the cell surface. The outer membrane of the cell envelope is a lipid bilayer with an asymmetric composition mostly of phospholipids in the inner leaflet and lipopolysaccha- rides (LPS) in the outer leaflet. The major membrane-forming phospholipid of Legionella spp. is phosphatidylcholine (PC)—a typical eukaryotic glycerophospholipid. PC synthesis in Legionella cells occurs via two independent pathways: the N-methylation (Pmt) pathway and the Pcs pathway. The utilisation of exogenous choline by Legionella spp. leads to changes in the composition of lipids and proteins, which influences the physicochemical properties of the cell surface. This phenotypic plastic- ity of the Legionella cell envelope determines the mode of interaction with the macrophages, which results in a decrease in the production of proinflammatory cytokines and modulates the interaction with antimicrobial peptides and proteins. The surface-exposed O-chain of Legionella pneumophila sg1 LPS consisting of a homopolymer of 5-acetamidino-7-acetamido-8-O-acetyl-3,5,7,9-tetradeoxy-L- glycero-D-galacto-non-2-ulosonic acid is probably the first component in contact with the host cell that anchors the bacteria in the host membrane. -

Lablink Winter 2014.Indd
Perit inci et, vel utpatum san- dio commy nit lore digna con eugueri ureros essi ea facil delismodiat, vel et augait ut wismod mod eliscilismod tion velis eugait augiat. Ut lut erae- strud mod molorercing ea con- sendre estrud. Vol. 19 No. 1 Winter 2014 Michigan Department of Community Health Bureau of Laboratories “Quality Laboratory Science for Healthier People and Communities” MDCH Bureau of Laboratories Announces Web Portal for Electronic Test Ordering and Results Delivery The Michigan Department of Community Health, Bureau of Laboratories (MDCH BOL), has instituted a web portal for Electronic Test Ordering and Results Delivery (ETOR). ETOR is an alternate, voluntary method for clients to order laboratory tests k and receive test results. Participating clients will be able to log into the web portal and electronically fill out test request forms; print a packing list for submission with samples to the MDCH BOL for testing; track sample progression through the MDCH laboratory; and look up, download, or print pdf copies of final reports. Clients using the web n portal will no longer have to hand write or submit test request forms. The information currently collected on the forms will be entered in the web portal and imported into the i BOL Laboratory Information Management System (LIMS) upon arrival of the specimen. Clients have the option to continue receiving final reports via fax or hard copy, as they do now. Web portal result delivery will also be available to the client. Upon request, any client may discontinue their fax or hard copy reporting system in favor of receiving only L web portal results. -

Legionella Feeleii: Pneumonia Or Pontiac Fever? Bacterial Virulence Traits and Host Immune Response
Medical Microbiology and Immunology (2019) 208:25–32 https://doi.org/10.1007/s00430-018-0571-0 REVIEW Legionella feeleii: pneumonia or Pontiac fever? Bacterial virulence traits and host immune response Changle Wang1 · Xia Chuai1 · Mei Liang2 Received: 17 July 2018 / Accepted: 27 October 2018 / Published online: 1 November 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Gram-negative bacterium Legionella is able to proliferate intracellularly in mammalian host cells and amoeba, which became known in 1976 since they caused a large outbreak of pneumonia. It had been reported that different strains of Legionella pneumophila, Legionella micdadei, Legionella longbeachae, and Legionella feeleii caused human respiratory diseases, which were known as Pontiac fever or Legionnaires’ disease. However, the differences of the virulence traits among the strains of the single species and the pathogenesis of the two diseases that were due to the bacterial virulence factors had not been well elucidated. L. feeleii is an important pathogenic organism in Legionellae, which attracted attention due to cause an outbreak of Pontiac fever in 1981 in Canada. In published researches, it has been found that L. feeleii serogroup 2 (ATCC 35849, LfLD) possess mono-polar flagellum, and L. feeleii serogroup 1 (ATCC 35072, WRLf) could secrete some exopolysaccharide (EPS) materials to the surrounding. Although the virulence of the L. feeleii strain was evidenced that could be promoted, the EPS might be dispensable for the bacteria that caused Pontiac fever. Based on the current knowledge, we focused on bacterial infection in human and murine host cells, intracellular growth, cytopathogenicity, stimulatory capacity of cytokines secre- tion, and pathogenic effects of the EPS ofL. -

Photoinactivation of Legionella Rubrilucens by Visible Light
European Journal of Microbiology and Immunology 7 (2017) 2, pp. 146–149 Original article DOI: 10.1556/1886.2017.00006 PHOTOINACTIVATION OF LEGIONELLA RUBRILUCENS BY VISIBLE LIGHT J. Schmid1, K. Hoenes1, M. Rath1, P. Vatter1, B. Spellerberg2, M. Hessling1,* 1 Ulm University of Applied Sciences, Albert-Einstein-Allee 55, D 89081 Ulm, Germany 2 Institute of Medical Microbiology and Hygiene, University of Ulm, Ulm, Germany Received: March 22, 2017; Accepted: March 28, 2017 In this study, the photoinactivation of Legionella by visible light is investigated. The success of this approach would offer new pros- pects for technical water disinfection and maybe even for therapeutic measures in cases of Legionella infections. Therefore, Legionella rubrilucens was dispensed on buffered charcoal yeast extract medium agar plates and illuminated with different doses of violet light generated by 405 nm light-emitting diodes (LEDs). A strong photoinactivation effect was observed. A dose of 125 J/ cm2 reduced the bacterial concentration by more than 5 orders of magnitude compared to Legionella on unirradiated agar plates. The necessary dose for a one log-level reduction was about 24 J/cm2. These results were obtained for extracellular L. rubri- lucens, but other Legionella species may exhibit a similar behavior. Keywords: Legionella rubrilucens, 405 nm irradiation, photoinactivation, disinfection Introduction disinfection effect on legionellae inside amoebae, almost twice the UV-C dose is required compared to planktonic Legionellae are ubiquitous in aquatic environments and legionellae. Usually, mercury vapor lamps are used as UV can therefore be found in natural reservoirs as well as in light sources for this kind of disinfection. -

Testing Different Membrane Filters for 16S Rrna Gene-Based Metabarcoding in Karstic Springs
Testing different membrane filters for 16S rRNA gene-based metabarcoding in karstic springs Oana Teodora Moldovan 1*, Andreea Baricz 2,3,4*, Edina Szekeres 2,3,4, Marius Kenesz 1, Marial Alexandra Hoaghia 5, Erika Andrea Levei 5, Ionuț Cornel Mirea 6, Ruxandra Năstase-Bucur 1, Traian Brad 1, Iulia Chiciudean 2,3, Horia Leonard Banciu 2,3 Figure S1. Piper diagram of the chemical elements for the studied springs Table S1. The relative abundance of the Bacteria phyla in the analyzed membranes, with the dominant Proteobacteria (see also Figure 3). BANPOTOC BAITA RAPOLTEL Phylum/Percentage AE20 AE21 AE22 AE23 AE24 AE25 AE26 AE4 AE27 BE11 BE12 BE18 BE16 BE15 BE13 BE19 BE17 BE14 CE29 CE31 CE32 CE30 CE34 CE33 CE2 CE28 CE35 CE3 non-identified 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 Acidobacteria 0,01 0,00 0,00 0,01 0,00 0,01 0,01 0,15 0,00 0,96 0,86 0,85 1,08 0,83 2,32 1,95 0,39 0,21 0,00 0,00 0,21 0,14 0,00 0,20 0,10 0,07 0,08 0,00 Actinobacteria 4,05 4,68 6,59 7,00 6,96 8,37 8,08 3,20 11,37 3,75 4,55 6,78 6,58 5,72 7,74 8,28 2,97 1,40 2,28 3,65 1,04 0,52 2,16 2,24 3,59 11,54 2,98 1,14 Aquificae 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 Armatimonadetes 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,01 0,01 0,00 0,00 0,00 0,01 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 0,00 Bacteroidetes 0,17 0,06 0,10 0,09 0,13 0,17 0,10 2,28 0,06 32,55