Volatile Variability and Antioxidant Activity of Rosmarinus Officinalis Essential Oil As Affected by Elevation Gradient and Vegetal Associations
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

State and Future of the Environment in the Oriental Region
Kingdom of Morocco Ministry of Energy, Mines, Ministry of Interior Water and Environment Region of Oriental Department of Environment Regional Observatory of Environment and Sustainable Development STATE AND FUTURE OF THE ENVIRONMENT IN THE ORIENTAL REGION Ministry of Energy, Mines, Water and Environment Department of Environment National Environmental Observatory of Morocco Adress : 9, Al Araar street, Sector 16, Hay Riyad, Rabat Phone : +212 (0) 5 37 57 66 41 Fax : +212 (0) 5 37 57 66 42 www.environnement.gov.ma Regional Observatory of Environment and Sustainable Development of the Oriental Region Adress : Siège du Conseil Régional, Bd, le Prince Héritier Moulay El Hassan , Oujda Phone : +212 (0) 5 36 52 48 70 SYNTHESIS REPORT FOR DECISION MAKERS Fax : +212 (0) 5 36 52 48 64 2013 Table of Contents THE ENVIRONMENTAL INTEGRATED ASSESSMENT, 06 01 A DECISION-MAKING TOOL 1.1 WHY THE NEED FOR A REGIONAL ENVIRONMENTAL INTEGRATED 06 ASSESSMENT? 1.2 A CONSULTATIVE AND PARTICIPATIVE APPROACH 06 A REGION WITH STRONG POTENTIAL, BUT WITH SIGNIFICANT 07 02 SOCIAL AND ENVIRONMENTAL ISSUES 2.1 A PREDOMINANTLY URBAN REGION 07 2.2 AN EMERGING ECONOMIC REGION 08 2.2.1 INDUSTRY 08 2.2.2 TRADING 09 2.2.3 AGRICULTURE AND LIVESTOCK 09 2.2.4 TOURISM 09 2.2.5 CRAFTMANSHIP 10 2.2.6 MINNING AND QUARRYING ACTIVITIES 10 2.2.7 SEA FISHING 11 2.2.8 TRANSPORTATION 11 03 ENVIRONMENTAL STATE AND TRENDS OF THE REGION 12 3.1 THE WORRYING FATE OF WATER RESSOURCES 12 3.1.1 QUANTITATIVE TERMS 12 3.1.2 QUALITATIVE TERMS 13 3.2 WASTEWATER SANITATION, AN ONGOING MANAGEMENT -

La Liste Des Jardins D'enfants Relevant Du Ministère De La Jeunesse Et Des Sports
la liste des Jardins d'Enfants relevant du Ministère de la Jeunesse et des Sports Délégation Etablissement Milieu Adresse Région : Rabat -Salé -Kenitra Rabat Alazhar Urbain Lotissement Alhaj Slimane n°15 C.Y.M Annahda Urbain Derb elfassi Rue joutiya n° 06 Souika Yaacoub eddar Urbain Rue 48 n° 1 Hay El Barid C.Y.M Provincial Urbain Avenue Sidi Mohamed Ben Abdellah C.Y.M Touarga Almichouar Said Touarga Urbain Almichouar Said Touarga Sale Tabrekte Urbain Hay Almazraä tabrekte Bettana Urbain Avenue Palestine Sale Achahbaa Urbain Avenue 2 Mars Rue Said Hajji Salé Alaayayda 1 Urbain Lgazzara Laayayda 1 Salé Alaayayda 2 Urbain Terrain Benaacher Alaayayda 2 Salé Skhirate Témara Almanzah Rural Commune Almanzah Sidi Yahya Rural Sidi Yahya zaër Ain Aaouda Urbain Eglise Ain Aaouda Annasr Urbain Hay Annasr Témara Hay Alfath Rural Hay Alfath skhirate Khémisset Provincial Urbain Avenue Idriss Elharti hay Essalam khémisset J.G Alaman Urbain Al hay alidari Khémisset Almahalli Urbain Rue Maarakate Badr Alkobra Khémisset Alamal Urbain Avenue la marche verte Tifelte Central « Almarkazi » Urbain N°66 rue almaazize hay alfarkh tifelte Albahraoui Rural - Arromani Rural Hay alamal Arromani Alghandour Rural Alghandour centre Alkanzara Rural Alkanzara centre Almaaziz Rural Almaaziz Alhay Alidari Ait Ikou Rural Ait Ikou centre Had Elbrachwa Rural Had Elbrachwa centre Ait Yadine Rural Ait Yadine centre Tiddasse Rural Tiddasse centre Khmisse Sidi Yahya Rural Commune Khmisse Sidi Yahya Oulmes Rural Oulmes centre Kénitra Alminaa Urbain Villa n°276 biir errami algharbiya -

Page 1 [email protected]
www.trackglobalsolutions.com [email protected] Notice: Procurement Notices Country: Morocco Sector: Water Financing: Agence Française de Développement Title: ACQUISITION DU MATERIEL D'EXPLOITATION ET D'ENTRETIEN POUR LES SERVICES D’ASSAINISSEMENT LIQUIDE POUR LES CENTRES DE : RAS EL MA – DRIOUCH – FARKHANA – MIDAR – EL AIOUNE/DEBDOU – TAGHAZOUT – LAKHSSAS - TEMSIA Project: Agence Française de Développement Date posted: 23 August 2017 Dead line: 05 October 2017 NOTICE ROYAUME DU MAROC OFFICE NATIONAL DE L’ELECTRICITE ET DE L’EAU POTABLE (ONEE) Branche Eau DIRECTION DES APPROVISIONNEMENTS ET MARCHES ACQUISITION DU MATERIEL D'EXPLOITATION ET D'ENTRETIEN POUR LES SERVICES D’ASSAINISSEMENT LIQUIDE POUR LES CENTRES DE : RAS EL MA – DRIOUCH – FARKHANA – MIDAR – EL AIOUNE/DEBDOU – TAGHAZOUT – LAKHSSAS - TEMSIA FOURNITURE DE MATERIELS DE SECURITE APPEL D'OFFRES OUVERT N° 28/DAM/AE/2017 Séance publique La Direction Approvisionnements et Marchés de l’ONEE-Branche Eau sise à Rabat, lance le présent Appel d'offres ouvert qui concerne l'acquisition du matériel d'exploitation et d'entretien pour les services d'assainissement liquide des centres de : RAS EL MA – DRIOUCH – FARKHANA – MIDAR – EL AIOUNE/DEBDOU – TAGHAZOUT – LAKHSSAS - TEMSIA, Fourniture de matériels de sécurité. Le financement du projet sera assuré par la coopération technique Belge (CTB). Les pièces justificatives à fournir sont celles prévues par l'article 10 du règlement de la consultation. L'estimation du coût des prestations s'élève à 1.019.994,00 DH TTC. Cette estimation reste indicative et ne constitue pas un montant maximum. Le montant du cautionnement provisoire est fixé à 10.200,00 DH (ou son équivalent en devise librement convertible). -

Construction.Pdf
Construction 2 TAILLE ET ÉVOLUTION DU MARCHÉ NOMBRE TOTAL D'ENTREPRISES (ACTIVES/ CRÉATIONS ANNUELLES INACTIVES) Année Maroc Orientale Nombre d’entreprises Maroc Orientale 2015 7 714 419 Global 119 007 7 119 2016 8 445 430 Statut actif 107 880 5 923 2017 8 196 391 Statut non actif 11 127 1 196 2018 9 585 435 2019 10 279 459 2020 6 616 336 1196 Entreprises Inactives Maroc 7714 6616 5923 Entreprises Actives Oriental 419 336 2015 2016 2017 2018 2019 2020 3 TAILLE ET ÉVOLUTION DU MARCHÉ RÉPARTITION GÉOGRAPHIQUE DES ENTREPRISES DE LA RÉGION L'ORIENTAL Province Ville Entreprises Berkane 757 Berkane Ahfir (M) 40 Berkane Aklim (M) 12 Berkane Berkane (M) 616 Berkane Boughriba 1 Berkane Chouihia 1 Berkane Fezouane 2 Berkane Laatamna 11 Berkane Madagh 18 Berkane Saidia (M) 71 Berkane Sidi Bouhria 1 Berkane Sidi Slimane Echcharraa (M) 6 Berkane Tafoughalt 1 Berkane Zegzel 5 Driouch 103 Driouch Ain Zohra 2 Driouch Azlaf 1 Driouch Ben Taieb 3 Driouch Dar El Kebdani 1 Driouch Driouch 80 Driouch Midar 8 Driouch Tafersit 1 Driouch Temsamane 6 Driouch Tsaft 1 Figuig 208 Figuig Bni Guil 3 Figuig Bni Tadjite 22 Figuig Bouanane 7 Figuig Bouarfa (M) 111 Figuig Figuig (M) 41 Figuig Talsint 22 Figuig Tendrara 3 Guercif 343 Guercif Guercif (M) 326 Guercif Houara Oulad Raho 4 Guercif Lamrija 2 Guercif Mazguitam 2 Guercif Ras Laksar 3 Guercif Saka 1 Guercif Taddart 8 Jerada 206 Jerada Ain Bni Mathar (M) 20 Jerada Gafait 4 Jerada Guenfouda 5 Jerada Jerada (M) 170 Jerada Lebkhata 1 Jerada Mrija 1 Jerada Touissit (M) 5 Nador 1 838 Nador Afsou 1 Nador Al Aaroui (M) -

Télécharger Le Document
CARTOGRAPHIE DU DÉVELOPPEMENT LOCAL MULTIDIMENSIONNEL NIVEAU ET DÉFICITS www.ondh.ma SOMMAIRE Résumé 6 Présentation 7 1. Approche méthodologique 8 1.1. Portée et lecture de l’IDLM 8 1.2. Fiabilité de l’IDLM 9 2. Développement, niveaux et sources de déficit 10 2.1. Cartographie du développement régional 11 2.2. Cartographie du développement provincial 13 2.3. Développement communal, état de lieux et disparité 16 3. L’IDLM, un outil de ciblage des programmes sociaux 19 3.1 Causes du déficit en développement, l’éducation et le niveau de vie en tête 20 3.2. Profil des communes à développement local faible 24 Conclusion 26 Annexes 27 Annexe 1 : Fiabilité de l’indice de développement local multidimensionnel (IDLM) 29 Annexe 2 : Consistance et méthode de calcul de l’indice de développement local 30 multidimensionnel Annexe 3 : Cartographie des niveaux de développement local 35 Annexes Communal 38 Cartographie du développement communal-2014 41 5 RÉSUMÉ La résorption ciblée des déficits socio-économiques à l’échelle locale (province et commune) requiert, à l’instar de l’intégration et la cohésion des territoires, le recours à une cartographie du développement au sens multidimensionnel du terme, conjuguée à celle des causes structurelles de son éventuel retard. Cette étude livre à cet effet une cartographie communale du développement et de ses sources assimilées à l’éducation, la santé, le niveau de vie, l’activité économique, l’habitat et les services sociaux, à partir de la base de données «Indicateurs du RGPH 2014» (HCP, 2017). Cette cartographie du développement et de ses dimensions montre clairement que : - La pauvreté matérielle voire monétaire est certes associée au développement humain, mais elle ne permet pas, à elle seule, d’identifier les communes sous l’emprise d’autres facettes de pauvreté. -

MPLS VPN Service
MPLS VPN Service PCCW Global’s MPLS VPN Service provides reliable and secure access to your network from anywhere in the world. This technology-independent solution enables you to handle a multitude of tasks ranging from mission-critical Enterprise Resource Planning (ERP), Customer Relationship Management (CRM), quality videoconferencing and Voice-over-IP (VoIP) to convenient email and web-based applications while addressing traditional network problems relating to speed, scalability, Quality of Service (QoS) management and traffic engineering. MPLS VPN enables routers to tag and forward incoming packets based on their class of service specification and allows you to run voice communications, video, and IT applications separately via a single connection and create faster and smoother pathways by simplifying traffic flow. Independent of other VPNs, your network enjoys a level of security equivalent to that provided by frame relay and ATM. Network diagram Database Customer Portal 24/7 online customer portal CE Router Voice Voice Regional LAN Headquarters Headquarters Data LAN Data LAN Country A LAN Country B PE CE Customer Router Service Portal PE Router Router • Router report IPSec • Traffic report Backup • QoS report PCCW Global • Application report MPLS Core Network Internet IPSec MPLS Gateway Partner Network PE Router CE Remote Router Site Access PE Router Voice CE Voice LAN Router Branch Office CE Data Branch Router Office LAN Country D Data LAN Country C Key benefits to your business n A fully-scalable solution requiring minimal investment -

Contrat-Progarmme Regional Region De L'oriental Au
Royaume du Maroc Ministère de l’Aménagement du Territoire National, de l’Urbanisme, de l’Habitat et de la Politique de la Ville CONTRAT-PROGARMME REGIONAL REGION DE L’ORIENTAL AU TITRE DE LA PERIODE 2017 - 2021 1 Oujda, le 27 Septembre 2017 Cadre référentiel Le présent Contrat - programme s’inscrit dans le cadre : des principes consacrés par la Constitution : en matière de bonne gouvernance : Les principes fondamentaux de la bonne gouvernance des services publics sont mis en exergue dans le Titre XII intitulé «De la bonne gouvernance - Principes généraux » ; en matière de planification stratégique, d’aménagement du territoire et d’urbanisme ; en matière de reddition des comptes cités plus particulièrement dans l’article 154 de la Constitution, et qui instaure clairement la liaison entre la responsabilité et la reddition des comptes ; en matière d’accès à un logement décent, article 31, qui stipule que : « L'Etat, les établissements publics et les collectivités territoriales œuvrent à la mobilisation de tous les moyens à disposition pour faciliter l'égal accès des citoyennes et des citoyens aux conditions leur permettant de jouir des droits :... à un logement décent au développement durable, à un environnement sain, … » ; en matière de suivi et évaluation ; principes cités dans plusieurs articles de la Constitution, notamment les articles 13-101-148 et 156 ; Des orientations Royales en matière d’Aménagement du Territoire National, d’Urbanisme, de l’Habitat et de la Politique de la ville ; Des engagements du programme gouvernemental -

Synthése Pour Les Décideurs
« Pour ce qui est du Maroc, nous avons créé, dès les années quatre-vingt dix du siècle passé, un observatoire national de l’environnement qui a pour mission de faire le suivi de la situation écologique de notre pays. Actuellement, nous sommes en passe de mettre sur pied des observatoires régionaux pour aider les collectivités locales à programmer leurs propres projets à partir de données environ- nementales précises et fiables. Cette action devrait également conforter le concept d’environ- nement de proximité » Extrait de la Lettre Royale, 3ème Congrès des Ministres de l’Environnement des pays de l’OCI, Octobre 2008 « Nous appelons le Gouvernement à élaborer un projet de Charte nationale globale de l’environnement, permettant la sauvegarde des espaces, des réserves et des ressources natu- relles, dans le cadre du processus de développement durable » Extrait du Discours du Trône, juillet 2009 « Aussi, engageons-Nous le gouvernement à donner corps aux grandes orientations issues du dialogue élargi visant l’élaboration d’une Charte nationale pour la protection de l’environ- nement et le développement durable, dans un plan d’action intégré ayant des objectifs précis et réalisables dans tous les secteurs d’activité. Parallèlement, Nous exhortons le gouvernement à formaliser ce plan dans un projet de loi-cadre, dont nous voulons qu’il constitue une véritable référence pour les politiques publiques de notre pays en la matière » Extrait du Discours du Trône, juillet 2010 Sa Majesté le Roi Mohammed VI, que Dieu l’assiste Table des Matières 08 L’ÉVALUATION -

The Jewish Heritage of Eastern Morocco Excerpt from the Royal Letter of February 13Th 2013 (By J.-M
Already published by La Croisé e des Chemins The Jewish Heritage BNI GUIL Espaces des Hommes Libres of Eastern Morocco The Jewish Heritage BNI GUIL Land of Free Men ISBN 978-9954-1-0393-7 of Eastern Morocco LES GRANDS ESPACES DE L’ORIENTAL MAROCAIN THE OPEN SPACES OF EASTERN MOROCCO ISBN 978-9954-1-0360-9 FIGUIG La ville oasis du Maroc oriental FIGUIG, The Oasis City of Eastern Morocco ISBN 978-9954-8924-1-9 À LA DÉ COUVERTE DE LA FAUNE DU MAROC ORIENTAL Itiné raires d’un naturaliste DISCOVERING EASTERN MOROCCO’S FAUNA Wanderings of a Naturalist “As is enshrined in the Kingdom’s new Constitution, the Hebrew ISBN 978-9954-1-0359-3 heritage is indeed one of the time-honored components of our national identity. For this reason, I wish to call for the restoration of all the www.lacroiseedeschemins.ma synagogues in the other Moroccan cities so that they may serve not only as places of worship, but also as forums for cultural dialogue and for the promotion of our cultural values.” Cover photo: The Merinid Kasbah of Debdou, and the surrounding countryside. The Jewish Heritage of Eastern Morocco Excerpt from the Royal letter of February 13th 2013 (by J.-M. Porte) Back cover, on the left: The tomb of Sidi Yahia in Oujda, a saint revered by ISBN. 978-9954-1-0517-7 Jews, Christians and Muslims. D.L. 2015 MO 0537 On the right: The Sefer Torah, a scroll of biblical texts still held in the Grand Synagogue of Oujda. www.oriental.ma (photos by M. -

Processing and Analysis of Aeromagnetic Data of North-Eastern
Processing and Analysis of aeromagnetic data of North-Eastern Morocco Hafsa Boufkri 1* , Driss El Azzab 1, Abdelhalim Miftah 2, Mohammed Charroud 1 1 Sidi Mohamed Ben Abdellah University, Faculty of Science and Technology, Laboratory of Intelligent Systems, Geo-resources and Renewable Energies, Fez, BP30050, Morocco. 2 Hassan First University of Settat, Faculty of Sciences and Technology, Research team of Geology of the Mining and Energetics Resources, BP577, Morocco. * Corresponding author: [email protected] 1 Abstract: The North-Eastern of Morocco was widely known for its mining potential, so discovering new structural guidelines was indispensable to find out new mineralization. Indeed, applying airborne magnetic techniques proves its efficiency in underlining new tectonic accidents and highlighting magnetic sources mostly hidden by Quaternary sedimentary covers. A magnetic anomaly map was established to attain this goal, basing on powerful operators (reduction to the pole, upward continuation, and Euler deconvolution), made on airborne data surveys of the study area. The elaborated map shows: (1) zones of strong magnetizations related to the Tertiary and Quaternary volcanic lavas partly outcropping in the Oujda and Saka regions to the iron and manganese mineralization concentrated in the Triassic beds. (2) Zones of relatively low magnetic response came from basaltic cones filled in a small graben at Oujda region. (3) Zones of low magnetization corresponded to the Quaternary and Tertiary cover or the Triassic-Jurassic deposits. We have established a magnetic lineaments map that emphasized deep faults, two main trends have been identified: NE-SW with ENE-WSW and E-W, they are considered as a major’s accidents because their depth reach to 2 km, as much as they inherited from the Hercynian and Alpine tectonics. -
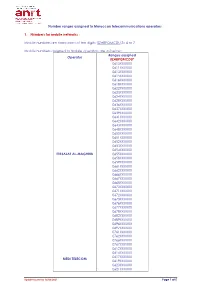
Number Ranges Assigned to Moroccan Telecommunications Operators
Number ranges assigned to Moroccan telecommunications operators 1. Numbers for mobile networks : Mobile numbers are composed of ten digits: 0ZABPQMCDU Z= 6 or 7. Mobile numbers assigned to mobile operators are as below: Ranges assigned Operator 0ZABPQMCDU* 0610XXXXXX 0611XXXXXX 0613XXXXXX 0615XXXXXX 0616XXXXXX 0618XXXXXX 0622XXXXXX 0623XXXXXX 0624XXXXXX 0628XXXXXX 0636XXXXXX 0637XXXXXX 0639XXXXXX 0641XXXXXX 0642XXXXXX 0643XXXXXX 0648XXXXXX 0650XXXXXX 0651XXXXXX 0652XXXXXX 0653XXXXXX 0654XXXXXX ITISSALAT AL-MAGHRIB 0655XXXXXX 0658XXXXXX 0659XXXXXX 0661XXXXXX 0662XXXXXX 0666XXXXXX 0667XXXXXX 0668XXXXXX 0670XXXXXX 0671XXXXXX 0672XXXXXX 0673XXXXXX 0676XXXXXX 0677XXXXXX 0678XXXXXX 0682XXXXXX 0689XXXXXX 0696XXXXXX 0697XXXXXX 0761XXXXXX 0762XXXXXX 0766XXXXXX 0767XXXXXX 0612XXXXXX 0614XXXXXX 0617XXXXXX MEDI TELECOM 0619XXXXXX 0620XXXXXX 0621XXXXXX Update issued on 14/06/2021 Page 1 of 5 0625XXXXXX 0631XXXXXX 0632XXXXXX 0644XXXXXX 0645XXXXXX 0649XXXXXX 0656XXXXXX 0657XXXXXX 0660XXXXXX 0663XXXXXX 0664XXXXXX 0665XXXXXX 0669XXXXXX 0674XXXXXX 0675XXXXXX 0679XXXXXX 0684XXXXXX 0688XXXXXX 0691XXXXXX 0693XXXXXX 0694XXXXXX 0770XXXXXX 0771XXXXXX 0772XXXXXX 0773XXXXXX 0774XXXXXX 0775XXXXXX 0777XXXXXX 0526XXXXXX 0527XXXXXX 0533XXXXXX 0534XXXXXX 0540XXXXXX 0546XXXXXX 0547XXXXXX 0550XXXXXX 0553XXXXXX 060XXXXXXX 0626XXXXXX 0627XXXXXX 0629XXXXXX 0630XXXXXX 0633XXXXXX 0634XXXXXX Wana Corporate 0635XXXXXX 0638XXXXXX 0640XXXXXX 0646XXXXXX 0647XXXXXX 0680XXXXXX 0681XXXXXX 0687XXXXXX 0690XXXXXX 0695XXXXXX 0698XXXXXX 0699XXXXXX 0700XXXXXX 0701XXXXXX 0702XXXXXX 0703XXXXXX -

Les Chrysomelidae Du Massif Du Debdou Dans Le Maroc (Coleoptera)
Les Chrysomelidae du massif du Debdou dans le Maroc (Coleoptera) PAR PIERRE JOLIVET. En attendant la parution d'une liste complète des Coléoptères du Debdou préparée par le Colonel Kocher, nous pensons qu'il est inté- ressant de faire le point de la faune chrysomélidienne de cette région, isolée et très caractéristique. Nous avons en, en effet, la possibilité de parcourir ce massif en compagnie de MM. W. Burssens, botaniste et B. E. Lapin, entomologiste, du 13 au 16 Mai 1967. Des récoltes intensives ont été effectuées tant sur le plateau que dans la plaine en- vironnante. Elles complètent la liste des espèces déj.à établie par Kocher. Nous remercions ici M. Kocher qui a examiné et déterminé nos récoltes. Dans la liste qui va suivre, nous nous efforcerons de donner quelques détails écologiques complémentaires. Les Coléoptères des autres familles seront étudiés à part. 1. LE MASSIF DU DEBDOU. CONSIDkRATIONS GÉOGRAPHIQUES ET CLI- MATIQUES. Les hautes plaines de Berguent (région où se rencontre encore le Timarcha punctella Marseul au Maroc oriental sont flanquées au Nord, de plis lourds, souvent faillés, qui prolongent le Moyen Atlas en deux rameaux dont l'un est constitué de chainons massifs qui alignent de Guercif-Taourirt à la frontière algérienne un chapelet de boutonnières paléozoiques, Debdou et Jerada qui sont dominées par des escarpe- ments calcaires de 1.500 1.700 m. Le deuxième rameau du Moyen At- las, plus septentrional est constitué de dómes discontinus depuis le Djebel Mezgout, à 1.900 ni., jusqu'aux Monts de Beni Snassem, 1.535 m. Les Monts de Tlemcen, de Daya, de Saida, de Trenda, en Algérie prolongent le Debdou et le Massif de Jerada vers l'Algérie.