Management of Endometriosis: an Enigmatic Disease
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Pushing Estrogen Receptor Around in Breast Cancer
Page 1 of 55 Accepted Preprint first posted on 11 October 2016 as Manuscript ERC-16-0427 1 Pushing estrogen receptor around in breast cancer 2 3 Elgene Lim 1,♯, Gerard Tarulli 2,♯, Neil Portman 1, Theresa E Hickey 2, Wayne D Tilley 4 2,♯,*, Carlo Palmieri 3,♯,* 5 6 1Garvan Institute of Medical Research and St Vincent’s Hospital, University of New 7 South Wales, NSW, Australia. 2Dame Roma Mitchell Cancer Research Laboratories 8 and Adelaide Prostate Cancer Research Centre, University of Adelaide, SA, 9 Australia. 3Institute of Translational Medicine, University of Liverpool, Clatterbridge 10 Cancer Centre, NHS Foundation Trust, and Royal Liverpool University Hospital, 11 Liverpool, UK. 12 13 ♯These authors contributed equally. *To whom correspondence should be addressed: 14 [email protected] or [email protected] 15 16 Short title: Pushing ER around in Breast Cancer 17 18 Keywords: Estrogen Receptor; Endocrine Therapy; Endocrine Resistance; Breast 19 Cancer; Progesterone receptor; Androgen receptor; 20 21 Word Count: 5620 1 Copyright © 2016 by the Society for Endocrinology. Page 2 of 55 22 Abstract 23 The Estrogen receptor-α (herein called ER) is a nuclear sex steroid receptor (SSR) 24 that is expressed in approximately 75% of breast cancers. Therapies that modulate 25 ER action have substantially improved the survival of patients with ER-positive breast 26 cancer, but resistance to treatment still remains a major clinical problem. Treating 27 resistant breast cancer requires co-targeting of ER and alternate signalling pathways 28 that contribute to resistance to improve the efficacy and benefit of currently available 29 treatments. -

(12) United States Patent (10) Patent No.: US 9,682,960 B2 Labrie Et Al
USOO968296OB2 (12) United States Patent (10) Patent No.: US 9,682,960 B2 Labrie et al. (45) Date of Patent: Jun. 20, 2017 (54) NON-STEROIDAL ANTANDROGENS AND (56) References Cited SELECTIVE ANDROGEN RECEPTOR MODULATORS WITH APYRIDYL MOETY U.S. PATENT DOCUMENTS 3,742.951 7, 1973 Zaffaroni (71) Applicants: Fernand Labrie, Quebec (CA); 3,797.494 3, 1974 Zaffaroni Shankar Mohan Singh, Quebec (CA); 4,568,343 2, 1986 Leeper et al. Richard Labrecque, St-Nicolas (CA); 5,064,654 11, 1991 Berner et al. 5,071,644 12, 1991 Viegas et al. Liviu Constantin Ciobanu, Quebec 5,071,657 12, 1991 Oloff et al. (CA) 5,411,981 5, 1995 Gaillard-Kelly et al. 5,556,983 9, 1996 Claussner et al. (72) Inventors: Fernand Labrie, Quebec (CA); 5,750,553 5, 1998 Claussner et al. 6,071,957 6/2000 Miller et al. Shankar Mohan Singh, Quebec (CA); 7/2000 Claussner et al. Richard Labrecque, St-Nicolas (CA); 6,087,509 Liviu Constantin Ciobanu, Quebec (Continued) (CA) FOREIGN PATENT DOCUMENTS (73) Assignee: ENDORECHERCHE, INC. (CA) CA 2 677 295 9, 2008 CA 2 773 591 3, 2011 EP 0 002892 A1 7/1979 (*) Notice: Subject to any disclaimer, the term of this EP O 100 172 A1 2, 1984 patent is extended or adjusted under 35 EP O 279 982 A1 8, 1988 U.S.C. 154(b) by 0 days. EP O 494 819 A1 7, 1992 EP O 578516 A1 1, 1994 Appl. No.: 14/567,970 EP O 580 459 A1 1, 1994 (21) FR 2 671 348 A1 7, 1992 Filed: Dec. -

WO 2018/144603 Al 09 August 2018 (09.08.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/144603 Al 09 August 2018 (09.08.2018) W !P O PCT (51) International Patent Classification: A61K 38/09 (2006 .01) A61P 35/00 (2006.0 1) (21) International Application Number: PCT/US20 18/0 16241 (22) International Filing Date: 31 January 2018 (3 1.01.2018) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/452,788 31 January 2017 (3 1.01.2017) US (71) Applicant: VERU INC. [US/US]; 4400 Biscayne Blvd., #888, Miami, Florida 33 137 (US). (72) Inventors: RAVI, Kacker; 39 Paul Revere Road, Lexing ton, Massachusetts 02421 (US). MITCHELL S., Stein- er; 2600 Forest Hill Road, Germantown, Tennessee 38139 (US). (74) Agent: COHEN, Mark S. et al; PEARL COHEN ZEDEK LATZER BARATZ LLP, 1500 Broadway, 12th Floor, New York, New York 10036 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Spectrum Pharmaceuticals Initiates US Clinical
Spectrum Pharmaceuticals Initiates U.S. Clinical Trial of Ozarelix (SPI-153) in Patients with Hormone-Dependent Prostate Cancer - Ozalerix is currently in two multicenter trials underway in Europe - one in benign prostate hypertrophy (BPH) and another in hormone dependent prostate cancer - Spectrum now has five drugs in multiple clinical trials, including one in phase III IRVINE, Calif., July 25 /PRNewswire-FirstCall/ -- Spectrum Pharmaceuticals, Inc. (Nasdaq: SPPI) today announced the launch of a phase I/II trial in the United States to explore the safe and efficacious dose range of ozarelix (formerly SPI-153) as a treatment for patients with hormone- dependent prostate cancer. "We are pleased to advance the development of ozarelix in the United States with the start of this clinical study," stated Rajesh Shrotriya, M.D., Chairman of the Board, Chief Executive Officer and President of Spectrum Pharmaceuticals, Inc. "This study will provide us additional valuable information regarding the optimum dose range for testosterone suppression, an important outcome measure in the management of hormone-dependent prostate cancer." About Prostate Cancer Prostate cancer is the second leading cause of cancer deaths in men and occurs when a malignant tumor forms in the tissue of the prostate. According to figures released by the American Cancer Society, approximately 232,090 new cases and 30,350 deaths will occur in the U.S. during 2005. The initial treatment of prostate cancer included surgery along with radiation therapy and hormonal therapy. About Ozarelix Ozarelix is a fourth generation LHRH (Luteinizing Hormone Releasing Hormone), also known as GnRH (Gonadotropin Releasing Hormone), antagonist. LHRH antagonists have the potential to treat hormone-dependent cancers as well as benign proliferative disorders such as benign prostatic hypertrophy and endometriosis. -
View Annual Report
Spectrum Pharmaceuticals, Inc. CANCER TAKES THE LIVES OF OVER SEVEN MILLION PEOPLE WORLDWIDE EACH YEAR www.spectrumpharm.com 157 Technology Drive, Irvine, California 92618 Telephone 949.788.6700 2005 Annual Report spECTRUM PHARMACEUTicALS, INC. SPECTRUM O U R T E A M PHARMACEUTicALS BOARD OF DIRECTORS MANAGEMENT TEAM OUTSIDE COUNSEL Rajesh C. Shrotriya, M.D. Rajesh C. Shrotriya, M.D. Latham & Watkins LLP Chairman of the Board, Chief Executive Officer Chairman, Chief Executive Officer & President Costa Mesa, California is COMMITTED TO & President, Spectrum Pharmaceuticals, Inc. Luigi Lenaz, M.D. I NDEPENDENT A UDITORS Richard D. Fulmer, M.B.A. Chief Scientific Officer Kelly & Company Former Vice President, Licensing and Development, Costa Mesa, California IMPROVING THE and Vice President of Marketing, Pfizer Inc. Shyam K. Kumaria, CPA, MBA Vice President, Finance T R A N S F E R A GENT Stuart M. Krassner, Sc.D., Ph.D. U.S. Stock Transfer Corporation LIVES OF PEOPLE Professor Emeritus of Developmental and Russell L. Skibsted Glendale, California Cell Biology at the School of Biological Sciences Senior Vice President, Chief Business Officer University of California at Irvine SEC F O R M 1 0 - K Ashok Y. Gore, Ph.D. Please see the enclosed Annual Report on Form WHO ARE FIGHTING Anthony E. Maida, III, M.A., M.B.A. Senior Vice President, Pharmaceutical 10-K filed with the Securities and Exchange Chairman, BioConsul Drug Development Operations & Regulatory Compliance Commission for a more detailed description Corporation and DendriTherapeutics, Inc. of the Company’s business, financial and other Consultant to various Venture Capital, William N. -

Medical Treatment for Adenomyosis And/Or Adenomyoma
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Taiwanese Journal of Obstetrics & Gynecology 53 (2014) 459e465 Contents lists available at ScienceDirect Taiwanese Journal of Obstetrics & Gynecology journal homepage: www.tjog-online.com Review Article Medical treatment for adenomyosis and/or adenomyoma Kuan-Hao Tsui a, b, 1, Wen-Ling Lee c, d, 1, Chih-Yao Chen a, e, Bor-Chin Sheu f, ** * Ming-Shyen Yen a, e, Ting-Chang Chang g, , Peng-Hui Wang a, e, h, i, j, a Department of Obstetrics and Gynecology, National Yang-Ming University School of Medicine, Taipei, Taiwan b Department of Obstetrics and Gynecology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan c Department of Medicine, Cheng-Hsin General Hospital, Taipei, Taiwan d Department of Nursing, Oriental Institute of Technology, New Taipei City, Taiwan e Division of Gynecology, Department of Obstetrics and Gynecology, Taipei Veterans General Hospital, Taipei, Taiwan f Department of Obstetrics and Gynecology, National Taiwan University Hospital and National Taiwan University, Taipei, Taiwan g Department of Obstetrics and Gynecology, Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan h Immunology Center, Taipei Veterans General Hospital, Taipei, Taiwan i Department of Medical Research, China Medical University Hospital, Taichung, Taiwan j Infection and Immunity Research, National Yang-Ming University, Taipei, Taiwan article info abstract Article history: Uterine adenomyosis and/or adenomyoma is characterized by the presence of heterotopic endometrial Accepted 9 April 2014 glands and stroma within the myometrium, >2.5 mm in depth in the myometrium or more than one microscopic field at 10 times magnification from the endometriumemyometrium junction, and a vari- Keywords: able degree of adjacent myometrial hyperplasia, causing globular and cystic enlargement of the myo- adenomyoma metrium, with some cysts filled with extravasated, hemolyzed red blood cells, and siderophages. -

Spectrum Announces Completion of Patient Enrollment in Ozarelix Phase II Trial in Patients with Benign Prostatic Hypertrophy
Spectrum Announces Completion of Patient Enrollment in Ozarelix Phase II Trial in Patients With Benign Prostatic Hypertrophy * Enrollment was completed ahead of schedule by more than four months * This is a multicenter, placebo controlled, randomized study in 144 patients with BPH IRVINE, Calif., Nov 21, 2005 /PRNewswire-FirstCall via COMTEX News Network/ -- Spectrum Pharmaceuticals, Inc. (Nasdaq: SPPI) today announced that the Company has achieved complete enrollment for a phase II trial of ozarelix in Benign Prostatic Hypertrophy (BPH), more that four months ahead of schedule. The multi-center clinical trial is designed to evaluate both objective parameters, such as improvement in urine flow and shrinkage of the prostate volume, as well as various symptoms of BPH over a period of several months. The trial is conducted in Europe with the collaboration of AEterna Zentaris Inc. (Nasdaq: AEZS; TSX: AEZ), "We are pleased to report that this multicenter, placebo controlled, randomized, phase II trial that was started only in April this year, has already achieved its goal of enrolling 144 patients, ahead of our projections by more than four months," stated Rajesh C. Shrotriya, M.D., Chairman, Chief Executive Officer and President. "Non-cancerous, enlargement of the prostate (BPH) adversely affects the quality of life, leading to increased frequency of urination and difficulty in passing urine. Because ozarelix is expected to have dual effects: (1), by directly shrinking the prostate and (2), through controlled reduction in the amount of testosterone (testosterone fuels the prostate gland), our expectation is that a single injection of ozarelix, repeated every few months, may be able to reduce the size of the prostate as well as accompanying symptoms." About Benign Prostatic Hypertrophy Benign prostatic hypertrophy is a non-cancerous enlargement of the prostate frequently occurring in men over the age of 50. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0190692 A1 VAN GROENINGHEN (43) Pub
US 20100190692A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0190692 A1 VAN GROENINGHEN (43) Pub. Date: Jul. 29, 2010 (54) METHODS FOR REDUCING Publication Classification GNRH-POSITIVE TUMIORCELL (51) Int. Cl. PROLIFERATION A638/09 (2006.01) (76) Inventor: JOHANNES C. VAN A638/22 (2006.01) GROENINGHEN, Dortmund (DE) A 6LX 3/59 (2006.01) s A63L/704 (2006.01) Correspondence Address: Age, 30.8; } GRUND INTELLECTUAL PROPERTY GROUP NKOLAISTRASSE 15 (52) U.S. Cl. .............. 514/8: 514/15; 514/259.5; 514/34; (57) ABSTRACT (21) Appl. No.: 12/701,284 A method for recognizing and evaluating the presence and (22) Filed: Feb. 5, 2010 function of GnRH receptors on tumor cells originating in the brain and/or nervous system and/or the meninges and/or reac Related U.S. Application Data tive neuroglia cells and/or primitive neuroectodermal tumor cells and/or on Kaposi sarcoma is provided. Furthermore a (63) Continuation-in-part of application No. 10/327,621, method for reducing degenerate GnRH-positive tumor cells filed on Dec. 20, 2002, now Pat. No. 7,695,722, which and/or for decreasing cellular replication of the above GnRH is a continuation-in-part of application No. 09/446, positive tumor cells comprising administering to a cell or to a 996, filed on Dec. 30, 1999, now abandoned, filed as Subject a replication decreasing amount of a GnRH agonist application No. PCT/DE98/01902 on Jul. 3, 1998. and/or GnRH antagonist and/or an erythropoietin agonist, and/or a thrombopoietin agonist, and/or a endothelin antago (30) Foreign Application Priority Data nist and/or a gonadotropin inhibiting hormone agonist is also provided. -

Expression Profiling of Nuclear Receptors in Breast Cancer Identifies TLX As a Mediator of Growth and Invasion in Triple-Negative Breast Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 6, No. 25 Expression profiling of nuclear receptors in breast cancer identifies TLX as a mediator of growth and invasion in triple-negative breast cancer Meng-Lay Lin1,*, Hetal Patel1,*, Judit Remenyi2, Christopher R. S. Banerji3,4, Chun-Fui Lai1, Manikandan Periyasamy1, Ylenia Lombardo1, Claudia Busonero1, Silvia Ottaviani1, Alun Passey1, Philip R. Quinlan5, Colin A. Purdie5, Lee B. Jordan5, Alastair M. Thompson6, Richard S. Finn7, Oscar M. Rueda8, Carlos Caldas8, Jesus Gil9, R. Charles Coombes1, Frances V. Fuller-Pace2, Andrew E. Teschendorff3,4, Laki Buluwela1, Simak Ali1 1 Department of Surgery & Cancer, Imperial College London, London, UK 2 Division of Cancer Research, University of Dundee, Ninewells Hospital & Medical School, Dundee, UK 3 Statistical Genomics Group, UCL Cancer Institute, University College London, London, UK 4 Centre of Mathematics and Physics in Life & Experimental Sciences, University College London, UK 5 Dundee Cancer Centre, Clinical Research Centre, University of Dundee, Ninewells Hospital & Medical School, Dundee, UK 6 Department of Surgical Oncology, MD Anderson Cancer Center, Houston, USA 7 Geffen School of Medicine at UCLA, Los Angeles, CA USA 8 Cancer Research UK Cambridge Institute, University of Cambridge Li Ka Shing Centre, Cambridge, UK 9 Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College London, Hammersmith Campus, London, UK * These authors have contributed equally to this work Correspondence to: Simak Ali, e-mail: [email protected] Keywords: cancer, nuclear receptors, expression profiling, breast cancer, tumour classification Received: March 11, 2015 Accepted: April 30, 2015 Published: May 13, 2015 ABSTRACT The Nuclear Receptor (NR) superfamily of transcription factors comprises 48 members, several of which have been implicated in breast cancer. -

(12) United States Patent (10) Patent No.: US 8,853,266 B2 Dalton Et Al
USOO8853266B2 (12) United States Patent (10) Patent No.: US 8,853,266 B2 Dalton et al. (45) Date of Patent: *Oct. 7, 2014 (54) SELECTIVE ANDROGEN RECEPTOR 3,875,229 A 4, 1975 Gold MODULATORS FOR TREATING DABETES 4,036.979 A 7, 1977 Asato 4,139,638 A 2f1979 Neri et al. 4,191,775 A 3, 1980 Glen (75) Inventors: James T. Dalton, Upper Arlington, OH 4,239,776 A 12/1980 Glen et al. (US): Duane D. Miller, Germantown, 4,282,218 A 8, 1981 Glen et al. TN (US) 4,386,080 A 5/1983 Crossley et al. 4411,890 A 10/1983 Momany et al. (73) Assignee: University of Tennessee Research 4,465,507 A 8/1984 Konno et al. F dati Kn ille, TN (US) 4,636,505 A 1/1987 Tucker Oundation, Knoxv1lle, 4,880,839 A 1 1/1989 Tucker 4,977,288 A 12/1990 Kassis et al. (*) Notice: Subject to any disclaimer, the term of this 5,162,504 A 11/1992 Horoszewicz patent is extended or adjusted under 35 5,179,080 A 1/1993 Rothkopfet al. U.S.C. 154(b) by 992 days. 5,441,868 A 8, 1995 Lin et al. 5,547.933 A 8, 1996 Lin et al. This patent is Subject to a terminal dis- 5,609,849 A 3/1997 Kung claimer. 5,612,359 A 3/1997 Murugesan et al. 5,618,698 A 4/1997 Lin et al. 5,621,080 A 4/1997 Lin et al. (21) Appl. No.: 11/785,064 5,656,651 A 8/1997 Sovak et al. -
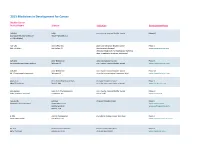
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor