Spectrum Announces Completion of Patient Enrollment in Ozarelix Phase II Trial in Patients with Benign Prostatic Hypertrophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,682,960 B2 Labrie Et Al
USOO968296OB2 (12) United States Patent (10) Patent No.: US 9,682,960 B2 Labrie et al. (45) Date of Patent: Jun. 20, 2017 (54) NON-STEROIDAL ANTANDROGENS AND (56) References Cited SELECTIVE ANDROGEN RECEPTOR MODULATORS WITH APYRIDYL MOETY U.S. PATENT DOCUMENTS 3,742.951 7, 1973 Zaffaroni (71) Applicants: Fernand Labrie, Quebec (CA); 3,797.494 3, 1974 Zaffaroni Shankar Mohan Singh, Quebec (CA); 4,568,343 2, 1986 Leeper et al. Richard Labrecque, St-Nicolas (CA); 5,064,654 11, 1991 Berner et al. 5,071,644 12, 1991 Viegas et al. Liviu Constantin Ciobanu, Quebec 5,071,657 12, 1991 Oloff et al. (CA) 5,411,981 5, 1995 Gaillard-Kelly et al. 5,556,983 9, 1996 Claussner et al. (72) Inventors: Fernand Labrie, Quebec (CA); 5,750,553 5, 1998 Claussner et al. 6,071,957 6/2000 Miller et al. Shankar Mohan Singh, Quebec (CA); 7/2000 Claussner et al. Richard Labrecque, St-Nicolas (CA); 6,087,509 Liviu Constantin Ciobanu, Quebec (Continued) (CA) FOREIGN PATENT DOCUMENTS (73) Assignee: ENDORECHERCHE, INC. (CA) CA 2 677 295 9, 2008 CA 2 773 591 3, 2011 EP 0 002892 A1 7/1979 (*) Notice: Subject to any disclaimer, the term of this EP O 100 172 A1 2, 1984 patent is extended or adjusted under 35 EP O 279 982 A1 8, 1988 U.S.C. 154(b) by 0 days. EP O 494 819 A1 7, 1992 EP O 578516 A1 1, 1994 Appl. No.: 14/567,970 EP O 580 459 A1 1, 1994 (21) FR 2 671 348 A1 7, 1992 Filed: Dec. -

WO 2018/144603 Al 09 August 2018 (09.08.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/144603 Al 09 August 2018 (09.08.2018) W !P O PCT (51) International Patent Classification: A61K 38/09 (2006 .01) A61P 35/00 (2006.0 1) (21) International Application Number: PCT/US20 18/0 16241 (22) International Filing Date: 31 January 2018 (3 1.01.2018) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/452,788 31 January 2017 (3 1.01.2017) US (71) Applicant: VERU INC. [US/US]; 4400 Biscayne Blvd., #888, Miami, Florida 33 137 (US). (72) Inventors: RAVI, Kacker; 39 Paul Revere Road, Lexing ton, Massachusetts 02421 (US). MITCHELL S., Stein- er; 2600 Forest Hill Road, Germantown, Tennessee 38139 (US). (74) Agent: COHEN, Mark S. et al; PEARL COHEN ZEDEK LATZER BARATZ LLP, 1500 Broadway, 12th Floor, New York, New York 10036 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Spectrum Pharmaceuticals Initiates US Clinical
Spectrum Pharmaceuticals Initiates U.S. Clinical Trial of Ozarelix (SPI-153) in Patients with Hormone-Dependent Prostate Cancer - Ozalerix is currently in two multicenter trials underway in Europe - one in benign prostate hypertrophy (BPH) and another in hormone dependent prostate cancer - Spectrum now has five drugs in multiple clinical trials, including one in phase III IRVINE, Calif., July 25 /PRNewswire-FirstCall/ -- Spectrum Pharmaceuticals, Inc. (Nasdaq: SPPI) today announced the launch of a phase I/II trial in the United States to explore the safe and efficacious dose range of ozarelix (formerly SPI-153) as a treatment for patients with hormone- dependent prostate cancer. "We are pleased to advance the development of ozarelix in the United States with the start of this clinical study," stated Rajesh Shrotriya, M.D., Chairman of the Board, Chief Executive Officer and President of Spectrum Pharmaceuticals, Inc. "This study will provide us additional valuable information regarding the optimum dose range for testosterone suppression, an important outcome measure in the management of hormone-dependent prostate cancer." About Prostate Cancer Prostate cancer is the second leading cause of cancer deaths in men and occurs when a malignant tumor forms in the tissue of the prostate. According to figures released by the American Cancer Society, approximately 232,090 new cases and 30,350 deaths will occur in the U.S. during 2005. The initial treatment of prostate cancer included surgery along with radiation therapy and hormonal therapy. About Ozarelix Ozarelix is a fourth generation LHRH (Luteinizing Hormone Releasing Hormone), also known as GnRH (Gonadotropin Releasing Hormone), antagonist. LHRH antagonists have the potential to treat hormone-dependent cancers as well as benign proliferative disorders such as benign prostatic hypertrophy and endometriosis. -
View Annual Report
Spectrum Pharmaceuticals, Inc. CANCER TAKES THE LIVES OF OVER SEVEN MILLION PEOPLE WORLDWIDE EACH YEAR www.spectrumpharm.com 157 Technology Drive, Irvine, California 92618 Telephone 949.788.6700 2005 Annual Report spECTRUM PHARMACEUTicALS, INC. SPECTRUM O U R T E A M PHARMACEUTicALS BOARD OF DIRECTORS MANAGEMENT TEAM OUTSIDE COUNSEL Rajesh C. Shrotriya, M.D. Rajesh C. Shrotriya, M.D. Latham & Watkins LLP Chairman of the Board, Chief Executive Officer Chairman, Chief Executive Officer & President Costa Mesa, California is COMMITTED TO & President, Spectrum Pharmaceuticals, Inc. Luigi Lenaz, M.D. I NDEPENDENT A UDITORS Richard D. Fulmer, M.B.A. Chief Scientific Officer Kelly & Company Former Vice President, Licensing and Development, Costa Mesa, California IMPROVING THE and Vice President of Marketing, Pfizer Inc. Shyam K. Kumaria, CPA, MBA Vice President, Finance T R A N S F E R A GENT Stuart M. Krassner, Sc.D., Ph.D. U.S. Stock Transfer Corporation LIVES OF PEOPLE Professor Emeritus of Developmental and Russell L. Skibsted Glendale, California Cell Biology at the School of Biological Sciences Senior Vice President, Chief Business Officer University of California at Irvine SEC F O R M 1 0 - K Ashok Y. Gore, Ph.D. Please see the enclosed Annual Report on Form WHO ARE FIGHTING Anthony E. Maida, III, M.A., M.B.A. Senior Vice President, Pharmaceutical 10-K filed with the Securities and Exchange Chairman, BioConsul Drug Development Operations & Regulatory Compliance Commission for a more detailed description Corporation and DendriTherapeutics, Inc. of the Company’s business, financial and other Consultant to various Venture Capital, William N. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0190692 A1 VAN GROENINGHEN (43) Pub
US 20100190692A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0190692 A1 VAN GROENINGHEN (43) Pub. Date: Jul. 29, 2010 (54) METHODS FOR REDUCING Publication Classification GNRH-POSITIVE TUMIORCELL (51) Int. Cl. PROLIFERATION A638/09 (2006.01) (76) Inventor: JOHANNES C. VAN A638/22 (2006.01) GROENINGHEN, Dortmund (DE) A 6LX 3/59 (2006.01) s A63L/704 (2006.01) Correspondence Address: Age, 30.8; } GRUND INTELLECTUAL PROPERTY GROUP NKOLAISTRASSE 15 (52) U.S. Cl. .............. 514/8: 514/15; 514/259.5; 514/34; (57) ABSTRACT (21) Appl. No.: 12/701,284 A method for recognizing and evaluating the presence and (22) Filed: Feb. 5, 2010 function of GnRH receptors on tumor cells originating in the brain and/or nervous system and/or the meninges and/or reac Related U.S. Application Data tive neuroglia cells and/or primitive neuroectodermal tumor cells and/or on Kaposi sarcoma is provided. Furthermore a (63) Continuation-in-part of application No. 10/327,621, method for reducing degenerate GnRH-positive tumor cells filed on Dec. 20, 2002, now Pat. No. 7,695,722, which and/or for decreasing cellular replication of the above GnRH is a continuation-in-part of application No. 09/446, positive tumor cells comprising administering to a cell or to a 996, filed on Dec. 30, 1999, now abandoned, filed as Subject a replication decreasing amount of a GnRH agonist application No. PCT/DE98/01902 on Jul. 3, 1998. and/or GnRH antagonist and/or an erythropoietin agonist, and/or a thrombopoietin agonist, and/or a endothelin antago (30) Foreign Application Priority Data nist and/or a gonadotropin inhibiting hormone agonist is also provided. -

(12) United States Patent (10) Patent No.: US 8,853,266 B2 Dalton Et Al
USOO8853266B2 (12) United States Patent (10) Patent No.: US 8,853,266 B2 Dalton et al. (45) Date of Patent: *Oct. 7, 2014 (54) SELECTIVE ANDROGEN RECEPTOR 3,875,229 A 4, 1975 Gold MODULATORS FOR TREATING DABETES 4,036.979 A 7, 1977 Asato 4,139,638 A 2f1979 Neri et al. 4,191,775 A 3, 1980 Glen (75) Inventors: James T. Dalton, Upper Arlington, OH 4,239,776 A 12/1980 Glen et al. (US): Duane D. Miller, Germantown, 4,282,218 A 8, 1981 Glen et al. TN (US) 4,386,080 A 5/1983 Crossley et al. 4411,890 A 10/1983 Momany et al. (73) Assignee: University of Tennessee Research 4,465,507 A 8/1984 Konno et al. F dati Kn ille, TN (US) 4,636,505 A 1/1987 Tucker Oundation, Knoxv1lle, 4,880,839 A 1 1/1989 Tucker 4,977,288 A 12/1990 Kassis et al. (*) Notice: Subject to any disclaimer, the term of this 5,162,504 A 11/1992 Horoszewicz patent is extended or adjusted under 35 5,179,080 A 1/1993 Rothkopfet al. U.S.C. 154(b) by 992 days. 5,441,868 A 8, 1995 Lin et al. 5,547.933 A 8, 1996 Lin et al. This patent is Subject to a terminal dis- 5,609,849 A 3/1997 Kung claimer. 5,612,359 A 3/1997 Murugesan et al. 5,618,698 A 4/1997 Lin et al. 5,621,080 A 4/1997 Lin et al. (21) Appl. No.: 11/785,064 5,656,651 A 8/1997 Sovak et al. -
Adis R&D Insight
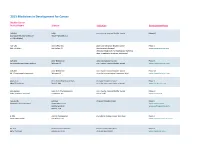
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor -

A61p5/28 (2006.01)
) ( (51) International Patent Classification: Declarations under Rule 4.17: A61K 31/4155 (2006.01) A61P5/28 (2006.01) — as to applicant's entitlement to apply for and be granted a A61K 31/4166 (2006.01) A61P 35/00 (2006.01) patent (Rule 4.17(H)) A61K 31/4439 (2006.01) — as to the applicant's entitlement to claim the priority of the (21) International Application Number: earlier application (Rule 4.17(iii)) PCT/IB2020/050752 Published: (22) International Filing Date: — with international search report (Art. 21(3)) 30 January 2020 (30.01.2020) — before the expiration of the time limit for amending the claims and to be republished in the event of receipt of (25) Filing Language: English amendments (Rule 48.2(h)) (26) Publication Language: English — in black and white; the international application as filed contained color or greyscale and is available for download (30) Priority Data: from PATENTSCOPE 62/798,836 30 January 2019 (30.01.2019) US 62/803,096 08 February 2019 (08.02.2019) US 62/822,3 12 22 March 2019 (22.03.2019) US 62/833,371 12 April 2019 (12.04.2019) US 62/836,920 22 April 2019 (22.04.2019) US 62/901,694 17 September 2019 (17.09.2019) US (71) Applicant: ARAGON PHARMACEUTICALS, INC. [US/US]; 10990 Wilshire Blvd, Suite 300, Los Angeles, California 90024 (US). (72) Inventor: YU, Margaret K.; 10990 Wilshire Blvd, Suite 300, Los Angeles, California 90024 (US). (74) Agent: SHIRTZ, Joseph F. et al.; Johnson & Johnson, One Johnson & Johnson Plaza, New Brunswick, New Jer¬ sey 08933 (US). -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

PEPTIDES in CANCER RESEARCH Cancer Research
PEPTIDES IN CANCER RESEARCH Cancer Research PEPTIDE-BASED CANCER THERAPEUTICS This brochure discusses the potential use of peptides as anti- cancer drugs highlighting current scenario and future prospects. Some peptides are also used as diagnostic tools for cancer detection. G-protein-coupled receptors are most important targets in drug development. Many of them are overexpressed in tumor cells. Amongst them, the GnRH receptor is the target of a consider- able number of GnRH agonists and antagonists used in cancer management. GnRH (gonadotropin-releasing hormone) or LHRH (luteinizing hormone-releasing hormone) is a decapeptide pro- duced in in the hypothalamus and released in a pulsatile fashion into the pituitary portal circulation. Prolonged non-pulsatile administration of LHRH leads to down-regulation of LH and FSH secretion, followed by a suppression of gonadal steroid synthesis. For this reason, longer-acting GnRH agonists as well as antago- nists are used for the treatment of hormone-dependent breast and prostate cancers. Most neuroendocrine tumors show a marked overexpression of somatostatin receptors, especially of sst2, which instigated the development of somatostatin agonists as octreotide. These compounds also play an important role in diagnosis. Bombesin/gastrin-releasing peptide receptors can be overex- pressed in malignant cells. Antagonists of these peptides inhibit tumor growth. Active immunization by peptide vaccines is another promising strategy to fight cancer. 2 Introduction penetrate tumor tissue. Their proteolytic Cancer is characterized by uncontrolled degradation can be conveniently prevented division of cells and the ability of these by chemical modifications such as incor- cells to invade other tissues leading to the poration of D-amino acids or cyclization. -

International Nonproprietary Names (Inn) for Biological and Biotechnological Substances
INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 15/06/2006 INTERNATIONAL NONPROPRIETARY NAMES (INN) FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES (A REVIEW) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines (QSM) Medicines Policy and Standards (PSM) Department CONTENTS 0. INTRODUCTION…………………………………….........................................................................................v 1. PHARMACOLOGICAL CLASSIFICATION OF BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………................................1 2. CURRENT STATUS OF EXISTING STEMS OR SYSTEMS FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES…………………….3 2.1 Groups with respective stems ……………………………………………………………………3 2.2 Groups with respective pre-stems………………………………………………………………4 2.3 Groups with INN schemes………………………………………………………………………….4 2.4 Groups without respective stems / pre-stems and without INN schemes…..4 3. GENERAL POLICIES FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………………………………………………………………………...5 3.1 General policies for blood products……………………………………………………………5 3.2 General policies for fusion proteins……………………………………………………………5 3.3 General policies for gene therapy products………………………………………………..5 3.4 General policies for glycosylated and non-glycosylated compounds………...6 3.5 General policies for immunoglobulins……………………………………………………….7 3.6 General polices for monoclonal antibodies………………………………………………..7 3.7 General polices for skin substitutes……………………………………………………………9 3.8 General policies for transgenic products……………………………………………………9 -

(INN) for Biological and Biotechnological Substances
WHO/EMP/RHT/TSN/2019.1 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) 2019 WHO/EMP/RHT/TSN/2019.1 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) 2019 International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) FORMER DOCUMENT NUMBER: INN Working Document 05.179 © World Health Organization 2019 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned.