THE GLOBAL DRACUNCULIASIS ERADICATION CAMPAIGN by Eric
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

MALI - Cercle De Gao: Carte De Référence (Octobre 2013)
MALI - Cercle de Gao: Carte de référence (Octobre 2013) KIDAL (KIDAL) Frontière internationale ALGERIE Limite de région Limite de cercle MAURITANIE Dabacar Chef-lieu de région Chef-lieu de cercle Kel amassine Kel takaraghate kalawate Icharamatane Chef-lieu de commune SENEGAL Kel taborack Village NIGER Kel sidalamine foulane Ilokane BURKINA FASO samit Cercle (autre région) Ifoghas GUINEE Kel bandaf 2 Cercle Gao Ibokalitane inouly Imrad divers MENAKA Forgeron Kel tafoulante Kel bandaf 1 Imilicha Ikefoutane COMMUNE Kel Imagrane talmen Inarwarene aboubakrine Ichadenharene Ikarbaganene Igawelene ANCHAWADI Kel Ighanagassane 1 tondibi GABERO Imagrane tikli Cheriffen haoussa BOUREM GAO Kel tanderbatene GOUNZOUREYE Ibohanane N'TILIT Kel ahad Imididaghane 2 SONI ALI BER Igorarene Ighanagassane 2 Imididaghane 1 TIILEMSI Kel Ikadeyane tadjalatt Cheriffen Fleuve Kel gourma TIILEMSI amdiliss Route principale Akodaka adinebangou Kareibandia Koygourou Badji Route tertiaire M'balde haoussa Kareibandia-ile Delega Goura Adineme Barissadji Meataha Aéroport international Kounsoum Barissadji Ile-goudelbaria Silwali Piste d'atterrissage Doumbaria SONI ALI BER Bagnadji Bossobon Gabame Lamboubero Hoyangaraba Djeboubero Kochakarei Kabanna DEMOGRAPHIE (2013) Baringouna AlizegameTondiagame Katia koima Seina ANCHAWADI Seyname Kel Total tanoukassane Taraykongo Berrahile Bella Ikalawatene cherifen kel doro GAO Ikayawane Chateau Kel razzaf Korogoussou Kosseye 8 472 157 8 472 157 16 806 999 Sidibe Mali Peulguelgobe Boulgoundie Bagoundie 2 Wabaria Norbene GOUNZOUREYE -

Lecture 5: Emerging Parasitic Helminths Part 2: Tissue Nematodes
Readings-Nematodes • Ch. 11 (pp. 290, 291-93, 295 [box 11.1], 304 [box 11.2]) • Lecture 5: Emerging Parasitic Ch.14 (p. 375, 367 [table 14.1]) Helminths part 2: Tissue Nematodes Matt Tucker, M.S., MSPH [email protected] HSC4933 Emerging Infectious Diseases HSC4933. Emerging Infectious Diseases 2 Monsters Inside Me Learning Objectives • Toxocariasis, larva migrans (Toxocara canis, dog hookworm): • Understand how visceral larval migrans, cutaneous larval migrans, and ocular larval migrans can occur Background: • Know basic attributes of tissue nematodes and be able to distinguish http://animal.discovery.com/invertebrates/monsters-inside- these nematodes from each other and also from other types of me/toxocariasis-toxocara-roundworm/ nematodes • Understand life cycles of tissue nematodes, noting similarities and Videos: http://animal.discovery.com/videos/monsters-inside- significant difference me-toxocariasis.html • Know infective stages, various hosts involved in a particular cycle • Be familiar with diagnostic criteria, epidemiology, pathogenicity, http://animal.discovery.com/videos/monsters-inside-me- &treatment toxocara-parasite.html • Identify locations in world where certain parasites exist • Note drugs (always available) that are used to treat parasites • Describe factors of tissue nematodes that can make them emerging infectious diseases • Be familiar with Dracunculiasis and status of eradication HSC4933. Emerging Infectious Diseases 3 HSC4933. Emerging Infectious Diseases 4 Lecture 5: On the Menu Problems with other hookworms • Cutaneous larva migrans or Visceral Tissue Nematodes larva migrans • Hookworms of other animals • Cutaneous Larva Migrans frequently fail to penetrate the human dermis (and beyond). • Visceral Larva Migrans – Ancylostoma braziliense (most common- in Gulf Coast and tropics), • Gnathostoma spp. Ancylostoma caninum, Ancylostoma “creeping eruption” ceylanicum, • Trichinella spiralis • They migrate through the epidermis leaving typical tracks • Dracunculus medinensis • Eosinophilic enteritis-emerging problem in Australia HSC4933. -
Summary of Protected Areas in Chad
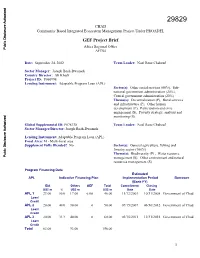
CHAD Community Based Integrated Ecosystem Management Project Under PROADEL GEF Project Brief Africa Regional Office Public Disclosure Authorized AFTS4 Date: September 24, 2002 Team Leader: Noel Rene Chabeuf Sector Manager: Joseph Baah-Dwomoh Country Director: Ali Khadr Project ID: P066998 Lending Instrument: Adaptable Program Loan (APL) Sector(s): Other social services (60%), Sub- national government administration (20%), Central government administration (20%) Theme(s): Decentralization (P), Rural services Public Disclosure Authorized and infrastructure (P), Other human development (P), Participation and civic engagement (S), Poverty strategy, analysis and monitoring (S) Global Supplemental ID: P078138 Team Leader: Noel Rene Chabeuf Sector Manager/Director: Joseph Baah-Dwomoh Lending Instrument: Adaptable Program Loan (APL) Focal Area: M - Multi-focal area Supplement Fully Blended? No Sector(s): General agriculture, fishing and forestry sector (100%) Theme(s): Biodiversity (P) , Water resource Public Disclosure Authorized management (S), Other environment and natural resources management (S) Program Financing Data Estimated APL Indicative Financing Plan Implementation Period Borrower (Bank FY) IDA Others GEF Total Commitment Closing US$ m % US$ m US$ m Date Date APL 1 23.00 50.0 17.00 6.00 46.00 11/12/2003 10/31/2008 Government of Chad Loan/ Credit APL 2 20.00 40.0 30.00 0 50.00 07/15/2007 06/30/2012 Government of Chad Loan/ Credit Public Disclosure Authorized APL 3 20.00 33.3 40.00 0 60.00 03/15/2011 12/31/2015 Government of Chad Loan/ Credit Total 63.00 93.00 156.00 1 [ ] Loan [X] Credit [X] Grant [ ] Guarantee [ ] Other: APL2 and APL3 IDA amounts are indicative. -

Relief Food Distribution in Mali
WORLD VISION ISTEKNATIONAL WORLD VISION RELIEF ORGANIZATION END OF PROJECT REPORT 1985 DROUGHT -RELIEF FOOD DISTRIBUTION IN MALI (T.A. 688-XXX-000-5622) (T.A. 641-XXX-000-5603) (P.A. 899-950-XXX-5784) Report prepared £or The United State Agency for International Development Mali Mission and AIDjW Office of Food for Peace March 1986 TABLE 'OF CONTENTS Page no. I INTRODUCTION 11. SUMMARY OF ACHIEVEMENTS AND CONSTRAINTS 111. TRANSPORT# STORAGE, LOSS AND DAMAGE I IV. DISTRIBUTION IN- THE 7TH REGION A. Project Personnel B. Logistics C. Start Up and Inter-Agency Coordination D. Population Surveys E. Distribution ' P'.. Distribution in Gao Ville G. Menaka Feeding Centers V. DISTRIBUTION IN NIORO A. Project Personnel B. Logistics C. Population Surveys D. Distribution VI. CONCLUSIONS AND RECOMMENDATIONS VII. SUMMARY TABLES OF DISTRIBUTIONS ANNEX - ACCOUNTINGI CONTROL AND REPORTING FORMS USED BY WV. ' ANNEX I. INTRODUCTION . World Vision International is a Christian humanitarian organiza- tion with the world headquarters in Monrovia, California, and currently active in major relief activities in four African nations using US PL480 food commodities. USAID requested the . World Vision Relief Organization to assist with the international response to the Malian drought in 1985 by distributing 10,000 metric tonnes of grain under a Direct Grant from the AID ~oodfor Peace Office. World Vision was to take full responsibility for transport and management of commodities from transfer of title in Ghana, through distribution in the target areas of Nioro du Sahel (1st Region), Gao, Menaka and Ansongo (7th region), Republic of Mali. The goal ofthe project wastoprevent starvationandstem the massive migration toward urban centers in these areas which had been severely affected by 5 years of inadequate rainfall. -

Pdf | 88.02 Kb
PRESS RELEASE MINUSMA strongly condemns the amputations of civilians in the Gao region and calls for the perpetrators to be brought to justice Bamako, 13 May 2021 – MINUSMA expresses its deep concern about the amputation on 2 May of the hands and feet of at least three civilian alleged road blockers, captured by suspected members of the Islamic State in the Great Sahara in Tin-Hama village, Ansongo cercle, Gao region. These developments are reminiscent of the horrors that marked the 2012 crisis and should be a wake-up call to all those involved in the fight against impunity in Mali. "I strongly condemn these despicable acts. Such corporal punishment carried out by armed groups outside any legal framework is a serious violation of human rights, including the right of every human being to a fair trial by a regularly constituted court. These abuses are punishable under Malian law", said the Special Representative of the Secretary- General in Mali (SRSG) and Head of MINUSMA, El-Ghassim Wane. While working to strengthen the fight against impunity in all its forms with the Malian authorities, MINUSMA recalls that attacks on physical integrity as well as being cruel, inhuman and degrading treatment, are in no way an acceptable solution under international law and are not constructive acts for justice and peace. "I reiterate MINUSMA's readiness to support the Malian authorities' ongoing investigations to combat impunity and ensure that the perpetrators of these acts are brought to justice", concluded Mr. Wane. MINUSMA, in accordance with its Mandate, is currently conducting a series of investigations into these allegations of serious human rights violations and is continuing its efforts to protect civilians by deploying significant security resources in the areas concerned to strengthen the protection of the population. -

Paper Submitted for Presentation at UNU-WIDER’S Conference, Held in Maputo on 5-6 July 2017
DRAFT WIDER Development Conference Public economics for development 5-6 July 2017 | Maputo, Mozambique This is a draft version of a conference paper submitted for presentation at UNU-WIDER’s conference, held in Maputo on 5-6 July 2017. This is not a formal publication of UNU-WIDER and may refl ect work-in-progress. THIS DRAFT IS NOT TO BE CITED, QUOTED OR ATTRIBUTED WITHOUT PERMISSION FROM AUTHOR(S). The impact of oil exploitation on wellbeing in Chad Abstract This study assesses the impact of oil revenues on wellbeing in Chad. Data used come from the two last Chad Household Consumption and Informal Sector Surveys ECOSIT 2 & 3 conducted in 2003 and 2011 by the National Institute of Statistics and Demographic Studies. A synthetic index of multidimensional wellbeing (MDW) is first estimated using a multiple components analysis based on a large set of welfare indicators. The Difference-in-Difference approach is then employed to assess the impact of oil revenues on the average MDW at departmental level. Results show that departments receiving intense oil transfers increased their MDW about 35% more than those disadvantaged by the oil revenues redistribution policy. Also, the farther a department is from the capital city N’Djamena, the lower its average MDW. Economic inclusion may be better promoted in Chad if oil revenues fit local development needs and are effectively directed to the poorest departments. Keys words: Poverty, Multidimensional wellbeing, Oil exploitation, Chad, Redistribution policy. JEL Codes: I32, D63, O13, O15 Authors Gadom -

Second Quarterly Report for Usg Fy 2011
Quarterly Report 1 July 2016 – 30 September 2016 IRTOUN “Rise Again” Funded by USAID / Office of Foreign Disaster Assistance (OFDA) Picture – Photo mosaic in Ansongo Cercle, photos by Field Team, Mercy Corps Annual Report Irtoun – Rise Again October 2015 – September 2016 1. Executive Summary With the support of USAID’s Office of Foreign Disaster Assistance (OFDA), Mercy Corps’ Irtoun program offers a package of integrated activities designed to enhance food security and economic resilience of communities recovering from the effects of conflict in Ansongo Circle of Gao Region and in Timbuktu and Gourma Rharous Circles of Timbuktu Region in Northern Mali. The program, initially funded for a period of 2 years from 11 February 2014-10 February 2016, was extended through two no- cost extensions through 30 September 2016, and received a cost modification to extend the project until 30 June 2017. At the end of FY16, the program has achieved or exceeded all of its objectives under the original implementation plan, and is in the start-up phase of the additional activities under the cost modification. During the quarterly reporting period, the program achieved the following: “Irtoun 1” Monitored and supported 45 village committees responsible for animal feed management to open accounts with microfinance institutions Awareness-raising of the population on best practices for animal feed harvesting and storage Advisory support and monitoring of the 22 veterinary assistants Follow-up with 53 micro-entrepreneurs on the management of their small enterprises Monitoring of VSLAs and village agents in the 4 communes of Ansongo “Irtoun 2” Kick-off meeting held in September 2016 for Irtoun II to review intervention strategy, budget, targets, timeline, procurement plan and staffing plan; Support to the 51 market gardening groups who participated in Irtoun I to prepare for the October 2016 vegetable planting season. -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

Public Health Significance of Intestinal Parasitic Infections*
Articles in the Update series Les articles de la rubrique give a concise, authoritative, Le pointfournissent un bilan and up-to-date survey of concis et fiable de la situa- the present position in the tion actuelle dans les do- Update selectedfields, coveringmany maines consideres, couvrant different aspects of the de nombreux aspects des biomedical sciences and sciences biomedicales et de la , po n t , , public health. Most of santepublique. Laplupartde the articles are written by ces articles auront donc ete acknowledged experts on the redigeis par les specialistes subject. les plus autorises. Bulletin of the World Health Organization, 65 (5): 575-588 (1987) © World Health Organization 1987 Public health significance of intestinal parasitic infections* WHO EXPERT COMMITTEE' Intestinal parasitic infections are distributed virtually throughout the world, with high prevalence rates in many regions. Amoebiasis, ascariasis, hookworm infection and trichuriasis are among the ten most common infections in the world. Other parasitic infections such as abdominal angiostrongyliasis, intestinal capil- lariasis, and strongyloidiasis are of local or regional public health concern. The prevention and control of these infections are now more feasible than ever before owing to the discovery of safe and efficacious drugs, the improvement and sim- plification of some diagnostic procedures, and advances in parasite population biology. METHODS OF ASSESSMENT The amount of harm caused by intestinal parasitic infections to the health and welfare of individuals and communities depends on: (a) the parasite species; (b) the intensity and course of the infection; (c) the nature of the interactions between the parasite species and concurrent infections; (d) the nutritional and immunological status of the population; and (e) numerous socioeconomic factors. -

IOM Nigeria DTM COVID-19 Point of Entry Dashboard (June 2020)
COVID-19 Point of Entry Dashboard: DTM North East Nigeria. Nigeria Monthly Snapshot June, 2020 Mamdi Barh-El-Gazel Ouest Wayi Mobbar Kukawa Lac Guzamala Dagana Dababa 45 766 Gubio Hadjer-Lamis Total movements (within, incoming and outgoing) Monguno Points of Entry Nganzai Ghana Haraze-Al-Biar observed Marte Magumeri Ngala N'Djamena 7 N'Djaména Yobe 164 Kala/Balge 13 OVERVIEW Jere Mafa Dikwa IOM DTM in collaboration with the World Health Organization (WHO) and the state Ministry of Health Maiduguri 13 Chari Kaga Chad Baguirmi have been conducting monitoring of individuals moving into Nigeria's conflict-affected northeastern Konduga Bama Chari-Baguirmi Bauchi states of Adamawa and Borno under pillar four (Points of entry) of COVID 19 preparedness and Borno Pulka Immigration Poe response planning guidelines. Gwoza Nigeria Damboa 29 211 Mayo-Lemié Chibok During the period 1 to 30 June 2020, 766 movements were observed at Forty Five Points of Entries in Biu Madagali Loug-Chari Extreme-Nord Adamawa and Borno states. Of the total movements recorded, 211 were incoming from Extreme-Nord, Askira/Uba Michika Mubi Road Kwaya Kusar Uba 34 from Nord, 6 from Centre in Cameroon and 13 from N’Djamena in Chad republic. A total of 264 Bayo Hawul Gombe 35 Mubi North Mayo-Boneye Incoming movements were observed at Seventeen Points of Entries. Bauchi HongMunduva Bahuli Shani Gombi BurhaKwaja Mayo-Kebbi Est 6 Kolere 4 Cameroon Shelleng Mayo-Binder A range of data was collected during the assessment to better inform on migrants’ nationalities, gender, Guyuk Song Maiha Mont Illi Bauchi Tashan Belel reasons for moving, mode of transportation and timeline of movement as shown in Figures 1 to 4 below: Adamawa 1 Tandjilé Est Lac Léré Lamurde 1 Kabbia Numan Girei Bilaci Tandjile Ouest Demsa Yola South Garin Dadi Mayo-Kebbi Ouest Tandjilé Kwarwa 34 Tandjilé Centre MAIN NATIONALITIES OBSERVED (FIG. -

Région Du GONTOUGO
RAPPORT DE SYNTHESE DU CONTROLE INOPINÉ DE LA QUALITÉ DE SERVICE (QoS) DES RÉSEAUX DE TÉLÉPHONIE MOBILE DANS LA REGION DU GONTOUGOU Du 14/06 au 14/09/2019 |www.valsch-consulting.com TABLE DES MATIERES CHAPITRE 1: CONTEXTE ET GENERALITES ................................................................................................ 3 1.1 INTRODUCTION ............................................................................................................................ 4 1.1.1 Contexte ...................................................................................................................................... 4 1.1.2 Objectifs de la mission .................................................................................................................. 4 1.1.3 Périmètre de la mission ................................................................................................................ 5 1.1.4 Services à auditer ......................................................................................................................... 7 1.2 Protocole de mesure et seuil de référence .................................................................................... 7 1.2.1 Evaluation du niveau de champs radioélectrique .......................................................................... 7 1.2.2 Evaluation du service voix en intra réseau ..................................................................................... 9 1.2.3 Evaluation de la qualité du service SMS ..................................................................................... -

Imaging Parasitic Diseases
Insights Imaging (2017) 8:101–125 DOI 10.1007/s13244-016-0525-2 REVIEW Unexpected hosts: imaging parasitic diseases Pablo Rodríguez Carnero1 & Paula Hernández Mateo2 & Susana Martín-Garre2 & Ángela García Pérez3 & Lourdes del Campo1 Received: 8 June 2016 /Revised: 8 September 2016 /Accepted: 28 September 2016 /Published online: 23 November 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Radiologists seldom encounter parasitic dis- • Some parasitic diseases are still endemic in certain regions eases in their daily practice in most of Europe, although in Europe. the incidence of these diseases is increasing due to mi- • Parasitic diseases can have complex life cycles often involv- gration and tourism from/to endemic areas. Moreover, ing different hosts. some parasitic diseases are still endemic in certain • Prompt diagnosis and treatment is essential for patient man- European regions, and immunocompromised individuals agement in parasitic diseases. also pose a higher risk of developing these conditions. • Radiologists should be able to recognise and suspect the This article reviews and summarises the imaging find- most relevant parasitic diseases. ings of some of the most important and frequent human parasitic diseases, including information about the para- Keywords Parasitic diseases . Radiology . Ultrasound . site’s life cycle, pathophysiology, clinical findings, diag- Multidetector computed tomography . Magnetic resonance nosis, and treatment. We include malaria, amoebiasis, imaging toxoplasmosis, trypanosomiasis, leishmaniasis, echino- coccosis, cysticercosis, clonorchiasis, schistosomiasis, fascioliasis, ascariasis, anisakiasis, dracunculiasis, and Introduction strongyloidiasis. The aim of this review is to help radi- ologists when dealing with these diseases or in cases Parasites are organisms that live in another organism at the where they are suspected.