The Decline of Fisheries Resources in New England
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cynoglossus Westraliensis, a New Species of Tonguesole from Western Australia (Teleostei: Cynoglossidae)
FishTaxa (2019) 4(2): 31-40 Journal homepage: www.fishtaxa.com © 2019 FISHTAXA. All rights reserved Cynoglossus westraliensis, a new species of tonguesole from Western Australia (Teleostei: Cynoglossidae) Ronald FRICKE* Im Ramstal 76, 97922 Lauda-Königshofen, Germany. Corresponding author: *E-mail: [email protected] Abstract The Western Australian deep-water tonguesole Cynoglossus westraliensis n. sp. is described from off North West Cape, Western Australia, based on two specimens collected at a depth of 250 metres. The new species is characterised within the Cynoglossus carpenteri species group by the snout relatively long, bluntly rounded; head length 21-25% of SL, snout length 9.3-11.6% of SL (43.4-46.5% of HL); eyes not contiguous; corner of mouth nearer to posterior edge of opercle than to tip of snout; ocular side with 3 lateral lines, midlateral-line scales 111-115, scale rows between midlateral and dorsolateral lines 20, blind side without lateral lines; ctenoid scales on ocular side, cycloid scales on blind side; dorsal-fin-rays 120-126; anal-fin rays 105-106; caudal-fin rays 8; gill chamber and peritoneum black. A key to the species of the Cynoglossus carpenteri species-group is presented. Keywords: Tonguesole, Cynoglossidae, Western Australia, New species, Identification key, Distribution. Zoobank: urn:lsid:zoobank.org:pub:C72DC2E5-9875-4DA0-9313-7967FB81C5C9 urn:lsid:zoobank.org:act:97081CA2-8CBA-4D70-8732-8BEA54017555 Introduction Tonguesoles of the family Cynoglossidae are small to medium sized benthic fishes, which are common in marine waters from tidal pools to the continental shelf and upper slope to a maximum depth of 1,500 m (Munroe 2001). -

Taxonomical Identification and Diversity of Flat Fishes from Mudasalodai Fish Landing Centre (Trawl by Catch), South East Coast of India
ISSN: 2642-9020 Review Article Journal of Marine Science Research and Oceanography Taxonomical Identification and Diversity of Flat Fishes from Mudasalodai Fish Landing Centre (Trawl by Catch), South East Coast of India Gunalan B* and E Lavanya *Corresponding author B Gunalan, PG & Research Department of Zoology, Thiru Kollanjiyapar PG & Research Department of Zoology, Thiru Kollanjiyapar Government Arts College, Viruthachalam. Cuddalore-Dt, Tamilnadu, India Government Arts College, Viruthachalam Submitted: 31 Jan 2020 Accepted: 05 Feb 2020; Published: 07 Mar 2020 Abstract Bycatch and discards are common and pernicious problems faced by all fisheries globally. It is recognized as unavoidable in any kind of fishing but the quantity varies according to the gear operated. In tropical countries like India, bycatch issue is more complex due to the multi-species and multi-gear nature of the fisheries. Among the different fishing gears, trawling accounts for a higher rate of bycatch, due to comparatively low selectivity of the gear. A study was conducted during June 2018 - Dec 2019 in the Mudasalodai fish landing centre, southeast coast of India. During the study period six sp. of flat fishes collected and identified taxonomically. Keywords: Flat fish, tongue fish, sole fish, bycatch, fish landing, waters of Parangipettai. The study was conducted for a period of diversity, taxonomy one and half year (June 2018 - Dec 2019), no sampling was done in the month of May, due to the fishing holiday in the coast of Introduction Tamil Nadu. The collected flat fishes were kept in ice boxes and Fish forms an important source of food and is man’s important transferred to the laboratory and washed in tap water. -

Food and Feeding Habits of Malabar Sole Cynoglossus Macrostomus Norman
J. mar. biol. Ass. India, 2000, 42 (1&2) : 124 - 134 Food and Feeding habits of Malabar sole Cynoglossus macrostomus Norman A. A. Jayaprakash Central Marine Fisheries Research Institute, Kochi - 682 014 Abstract The food and feeding habits of Malabar sole Cynoglossus macrostomus Norman occurring along the coastal seas off Kerala were studied both qualitatively and quantitatively during 1994-96. The samples for the study were collected from three widely located centres like Cochin, Ambalapuzha and Neendakara. The fish mainly adapted to a bottom habitat feeds on polychaetes and detritus, amphipods, copepods, small molluscs and foraminifera. Active feed- ing was found immediately after spawining during October - November and February March. There was not much difference in the forage items noticed at different centres. Since the detritus is an important food component followed by other macrobenthos the fish can safely be placed between trophic level I and 11. Introduction Cynoglossus lingua; Devadoss and Pillai Investigations on the food and feeding (1973), Devadoss et a1 (1977), Ramanathan habits of fishes have traditionally been and Natarajan (1980) and Ramanathan important in fishery biological studies since et a1 (1977) on other flatfish species. food is one of the key factors that pro- Among the flatfishes, only few species foundly influence the shoaling, behaviour, like the Malabar sole support a fishery of migration, condition and even the fishery. commercial importance. The species con- The Pleuronectids, comprising the flatfishes tributed nearly 95% of the total 25,000 t by virtue of their body form and bottom of flatfishes landed in Kerala. Apart from habitat have attracted the attention of the studies on the food and feeding habits many workers. -

Assessing the Impact of Key Marine Invasive Non-Native Species on Welsh MPA Habitat Features, Fisheries and Aquaculture
Assessing the impact of key Marine Invasive Non-Native Species on Welsh MPA habitat features, fisheries and aquaculture. Tillin, H.M., Kessel, C., Sewell, J., Wood, C.A. Bishop, J.D.D Marine Biological Association of the UK Report No. 454 Date www.naturalresourceswales.gov.uk About Natural Resources Wales Natural Resources Wales’ purpose is to pursue sustainable management of natural resources. This means looking after air, land, water, wildlife, plants and soil to improve Wales’ well-being, and provide a better future for everyone. Evidence at Natural Resources Wales Natural Resources Wales is an evidence based organisation. We seek to ensure that our strategy, decisions, operations and advice to Welsh Government and others are underpinned by sound and quality-assured evidence. We recognise that it is critically important to have a good understanding of our changing environment. We will realise this vision by: Maintaining and developing the technical specialist skills of our staff; Securing our data and information; Having a well resourced proactive programme of evidence work; Continuing to review and add to our evidence to ensure it is fit for the challenges facing us; and Communicating our evidence in an open and transparent way. This Evidence Report series serves as a record of work carried out or commissioned by Natural Resources Wales. It also helps us to share and promote use of our evidence by others and develop future collaborations. However, the views and recommendations presented in this report are not necessarily those of -

Witch Flounder, Glyptocephalus Cynoglossus, Life History and Habitat Characteristics
NOAA Technical Memorandum NMFS-NE-139 Essential Fish Habitat Source Document: Witch Flounder, Glyptocephalus cynoglossus, Life History and Habitat Characteristics U. S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Northeast Region Northeast Fisheries Science Center Woods Hole, Massachusetts September 1999 Recent Issues 105. Review of American Lobster (Homarus americanus) Habitat Requirements and Responses to Contaminant Exposures. By Renee Mercaldo-Allen and Catherine A. Kuropat. July 1994. v + 52 p., 29 tables. NTIS Access. No. PB96-115555. 106. Selected Living Resources, Habitat Conditions, and Human Perturbations of the Gulf of Maine: Environmental and Ecological Considerations for Fishery Management. By Richard W. Langton, John B. Pearce, and Jon A. Gibson, eds. August 1994. iv + 70 p., 2 figs., 6 tables. NTIS Access. No. PB95-270906. 107. Invertebrate Neoplasia: Initiation and Promotion Mechanisms -- Proceedings of an International Workshop, 23 June 1992, Washington, D.C. By A. Rosenfield, F.G. Kern, and B.J. Keller, comps. & eds. September 1994. v + 31 p., 8 figs., 3 tables. NTIS Access. No. PB96-164801. 108. Status of Fishery Resources off the Northeastern United States for 1994. By Conservation and Utilization Division, Northeast Fisheries Science Center. January 1995. iv + 140 p., 71 figs., 75 tables. NTIS Access. No. PB95-263414. 109. Proceedings of the Symposium on the Potential for Development of Aquaculture in Massachusetts: 15-17 February 1995, Chatham/Edgartown/Dartmouth, Massachusetts. By Carlos A. Castro and Scott J. Soares, comps. & eds. January 1996. v + 26 p., 1 fig., 2 tables. NTIS Access. No. PB97-103782. 110. Length-Length and Length-Weight Relationships for 13 Shark Species from the Western North Atlantic. -
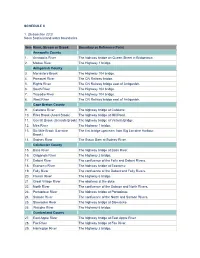
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

FOR REFERENCE USE ONL Y DO NOT Removt from LIBRARY
Canada. F isbcries Service Maritimes Region. R csource Development Branch MANUSCRIPT REPORT I+ Environment Canada Environnement Canada RESOURCE DEVELOPMENT BRANCH .. " ' IÎÏI ~ Îl ~l Ïl\ij fü1imÎ l~I Îl \ i1 li~ ~Î~Ïil l I 'J 09093266 A Preliminary Investigation of the Striped Bass, Roccus saxatilis, Fishery Resource in the Annapolis River System, and the General Distribution of Striped Bass in Other Areas in Southwestern Nova Scotia by G. H. PENNEY FOR REFERENCE USE ONL Y DO NOT REMOvt FROM LIBRARY f h~trlts Stnlct 11111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111 Hallfa1, N.S. 1l7d.- Restricted MANUSCRIPT REPORT No. 73- 4 A PRELIMINARY INVESTIGATION OF THE STRIPED BASS, Roccus saxatilis ~ FISHERY RESOURCE IN THE ANNAPOLIS RIVER SYSTEM, AND THE GENERAL DISTRIBUTION OF STRIPED BASS IN OTHER AREAS IN SOUTHWESTERN NOVA SCOTIA BY G.H. PENNEY Restricted A PRELIMINARY INVESTIGATION OF THE STRIPED BASS, Roccus s axatilis , FISHERY RESOURCE IN THE ANNAPOLIS RIVER SYSTEM, AND THE GENERAL DISTRIBUTION OF STRIPED BASS IN OTHER AREAS IN SOUTHWESTERN NOVA SCOTIA BY G.H. PENNEY DEPARTMENT OF THE ENVIRONMENT FISHERIES SERVICE RESOURCE DEVELOPMENT BRANCH HALIFAX, NOVA SCOTIA MARCH, 1973. CONTENTS PAGE INTRODUCTION . • . • . • . • . • . • 1 METHODS 8 RESULTS 10 (a) Angling Statistics (1951-1972)-Annapolis River system . ....................................... 10 (b) Length, Weight, Sex, and Age of Striped Bass sampled from the Annapolis River in 1972 .....• 12 (c) Residence Distribution of Anglers from which Samples were obtained ........................ 16 (d) Angling Pressure for Striped Bass during June, July, and August, 1972, at the Annapolis 18 Causeway ..............•....................... (e) General Distribution and Abundance of Striped Bass in Other Areas in Southwestern Nova 20 Scotia ....................................... DISCUSSION - RECOMMENDATIONS ....................... -

South Western Nova Scotia
Netukulimk of Aquatic Natural Life “The N.C.N.S. Netukulimkewe’l Commission is the Natural Life Management Authority for the Large Community of Mi’kmaq /Aboriginal Peoples who continue to reside on Traditional Mi’Kmaq Territory in Nova Scotia undisplaced to Indian Act Reserves” P.O. Box 1320, Truro, N.S., B2N 5N2 Tel: 902-895-7050 Toll Free: 1-877-565-1752 2 Netukulimk of Aquatic Natural Life N.C.N.S. Netukulimkewe’l Commission Table of Contents: Page(s) The 1986 Proclamation by our late Mi’kmaq Grand Chief 4 The 1994 Commendation to all A.T.R.A. Netukli’tite’wk (Harvesters) 5 A Message From the N.C.N.S. Netukulimkewe’l Commission 6 Our Collective Rights Proclamation 7 A.T.R.A. Netukli’tite’wk (Harvester) Duties and Responsibilities 8-12 SCHEDULE I Responsible Netukulimkewe’l (Harvesting) Methods and Equipment 16 Dangers of Illegal Harvesting- Enjoy Safe Shellfish 17-19 Anglers Guide to Fishes Of Nova Scotia 20-21 SCHEDULE II Specific Species Exceptions 22 Mntmu’k, Saqskale’s, E’s and Nkata’laq (Oysters, Scallops, Clams and Mussels) 22 Maqtewe’kji’ka’w (Small Mouth Black Bass) 23 Elapaqnte’mat Ji’ka’w (Striped Bass) 24 Atoqwa’su (Trout), all types 25 Landlocked Plamu (Landlocked Salmon) 26 WenjiWape’k Mime’j (Atlantic Whitefish) 26 Lake Whitefish 26 Jakej (Lobster) 27 Other Species 33 Atlantic Plamu (Salmon) 34 Atlantic Plamu (Salmon) Netukulimk (Harvest) Zones, Seasons and Recommended Netukulimk (Harvest) Amounts: 55 SCHEDULE III Winter Lake Netukulimkewe’l (Harvesting) 56-62 Fishing and Water Safety 63 Protecting Our Community’s Aboriginal and Treaty Rights-Community 66-70 Dispositions and Appeals Regional Netukulimkewe’l Advisory Councils (R.N.A.C.’s) 74-75 Description of the 2018 N.C.N.S. -

Identification of the Sole Resources of the Gambia
Identification of the Sole Resources of The Gambia Gambia-Senegal Sustainable Fisheries Program (Ba Nafaa) December 2011 This publication is available electronically on the Coastal Resources Center’s website at http://www.crc.uri.edu. For more information contact: Coastal Resources Center, University of Rhode Island, Narragansett Bay Campus, South Ferry Road, Narragansett, Rhode Island 02882, USA. Tel: 401) 874-6224; Fax: 401) 789-4670; Email: [email protected] The BaNafaa project is implemented by the Coastal Resources Center of the University of Rhode Island and the World Wide Fund for Nature-West Africa Marine Ecoregion (WWF-WAMER) in partnership with the Department of Fisheries and the Ministry of Fisheries, Water Resources and National Assembly Matters. Citation: Coastal Resources Center, 2011. Identification of the Sole Resources of The Gambia. Coastal Resources Center, University of Rhode Island, pp.11 Disclaimer: This report was made possible by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. Cooperative Agreement # 624-A-00-09- 00033-00. Cover Photo: Coastal Resources Center/URI Fisheries Center Photo Credit: Coastal Resources Center/URI Fisheries Center 2 The Sole Resources Proper identification of the species is critical for resource management. There are four major families of flatfish with representative species found in the Gambian nearshore waters: Soleidae, Cynoglossidae, Psettododae and Paralichthyidae. The species below have been confirmed through literature review, and through discussions with local fishermen, processors and the Gambian Department of Fisheries. -

A River Runs Through It: an Archaeological Survey of the Upper
Library and Bibliotheque et 1*1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-47882-0 Our file Notre reference ISBN: 978-0-494-47882-0 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a la Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par Plntemet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. this thesis. Neither the thesis Ni la these ni des extraits substantiels de nor substantial extracts from it celle-ci ne doivent etre imprimes ou autrement may be printed or otherwise reproduits sans son autorisation. reproduced without the author's permission. In compliance with the Canadian Conformement a la loi canadienne Privacy Act some supporting sur la protection de la vie privee, forms may have been removed quelques formulaires secondaires from this thesis. ont ete enleves de cette these. -

Contents of Volume 43 (2013)
CONTENTS OF VOLUME 43 (2013) Issue 1 Tsikliras A.C., Stergiou K.I., Froese R. Editorial note on reproductive biology of fishes .......................................................................................................................................................... 1 Thulasitha W.S., Sivashanthini K. Reproductive characteristics of doublespotted queenfish, Scomberoides lysan (Actinopterygii: Perciformes: Carangidae), from Sri Lankan waters: Implications for fisheries management ................... 7 Rajkumar M., Rahman M.M., Reni Prabha A., Phukan B. Effect of cholymbi on growth, proximate composition, and digestive enzyme activity of fingerlings of long whiskered catfish, Mystus gulio (Actinopterygii: Siluriformes: Bagridae) ........................................................................... 15 Muchlisin Z.A., Thomy Z., Fadli N., Sarong M.A., Siti-Azizah M.N. DNA barcoding of freshwater fishes from Lake Laut Tawar, Aceh Province, Indonesia ............................................................. 21 Kırankaya Ş.G., Ekmekçi F.G. Life-history traits of the invasive population of Prussian carp, Carassius gibelio (Actinopterigi: Cypriniformes: Cyprinidae), from Gelingüllü Reservoir, Yozgat, Turkey .......................................................... 31 Pan T., Yan M., Chen S., Wang X. Effects of ten traditional Chinese herbs on immune response and disease resistance of Sciaenops ocellatus (Actinopterygii: Perciformes: Sciaenidae) ................................................................................................................................................................... -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate.