Dissecting the Circuitry of the Auditory System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -

Neuronal Organization in the Inferior Colliculus Revisited with Cell-Type- Dependent Monosynaptic Tracing
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Neuronal organization in the inferior colliculus revisited with cell-type- dependent monosynaptic tracing Chenggang Chen1, Mingxiu Cheng1,2, Tetsufumi Ito3 and Sen Song1 1Tsinghua Laboratory of Brain and Intelligence (THBI) and Department of Biomedical Engineering, Beijing Innovation Center for Future Chip, Center for Brain-Inspired Computing Research, McGovern Institute for Brain Research, Tsinghua University, Beijing, 100084, China 2National Institute of Biological Sciences, Beijing, 102206, China 3Anatomy II, School of Medicine, Kanazawa Medical University, Uchinada, Ishikawa, 920-0293, Japan DOI: 10.1523/JNEUROSCI.2173-17.2018 Received: 31 July 2017 Revised: 2 February 2018 Accepted: 7 February 2018 Published: 24 February 2018 Author contributions: C.C., T.I., and S.S. designed research; C.C. and M.C. performed research; C.C. and T.I. analyzed data; C.C. wrote the first draft of the paper; C.C., T.I., and S.S. edited the paper; C.C., T.I., and S.S. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. This work was supported by funding from the National Natural Science Foundation of China (31571095, 91332122, for S.S.), Special Fund of Suzhou-Tsinghua Innovation Leading Action (for S.S.), Beijing Program on the Study of Brain-Inspired Computing System and Related Core Technologies (for S.S.), Beijing Innovation Center for Future Chip (for S.S.), and Chinese Academy of Sciences Institute of Psychology Key Laboratory of Mental Health Open Research Grant (KLMH2012K02, for S.S.), grants from Ministry of Education, Science, and Culture of Japan (KAKENHI grant, Grant numbers 16K07026 and 16H01501; for T.I.), and Takahashi Industrial and Economic Research Foundation (for T.I.). -

MR Imaging of Primary Trochlear Nerve Neoplasms
707 MR Imaging of Primary Trochlear Nerve Neoplasms Lindell R. Gentry 1 We present the clinical, anatomic, and MR imaging findings in six patients with seven Rahul C. Mehta 1 primary trochlear nerve neoplasms, as well as the MR and clinical criteria that serve to Richard E. Appen2 establish the diagnosis of these rare cranial nerve neoplasms. Three patients had a Joel M. Weinstein2 history of neurofibromatosis and five patients had clinical evidence of a trochlear nerve palsy. Six of seven neoplasms produced localized, fusiform enlargement of the proximal cisternal segments of the trochlear nerves. The lesions that were visible on noncontrast MR scans (T1-, T2-, and proton density-weighted) had signal intensities that were virtually identical to normal brain parenchyma. All lesions showed intense, homogeneous enhancement on contrast-enhanced scans. Contrast-enhanced imaging was necessary for the detection of five of seven lesions and greatly increased the value of the MR study in all six patients. AJNR 12:707-713, July/August 1991; AJR 157: September 1991 Primary neoplasms arising from pure motor cranial nerves such as the trochlear nerve are rare. To our knowledge just nine primary trochlear nerve neoplasms have been reported in the literature [1-7), only one of which was imaged with MR [1). Over the last 6 years, since we began to use MR as the primary imaging method for evaluating patients with cranial nerve palsies , we have noticed a striking increase in the number of primary trochlear nerve neoplasms over those encountered during the CT era. We believe this is due to a much greater sensitivity of MR in detecting these typically small lesions. -

Auditory and Vestibular Systems Objective • to Learn the Functional
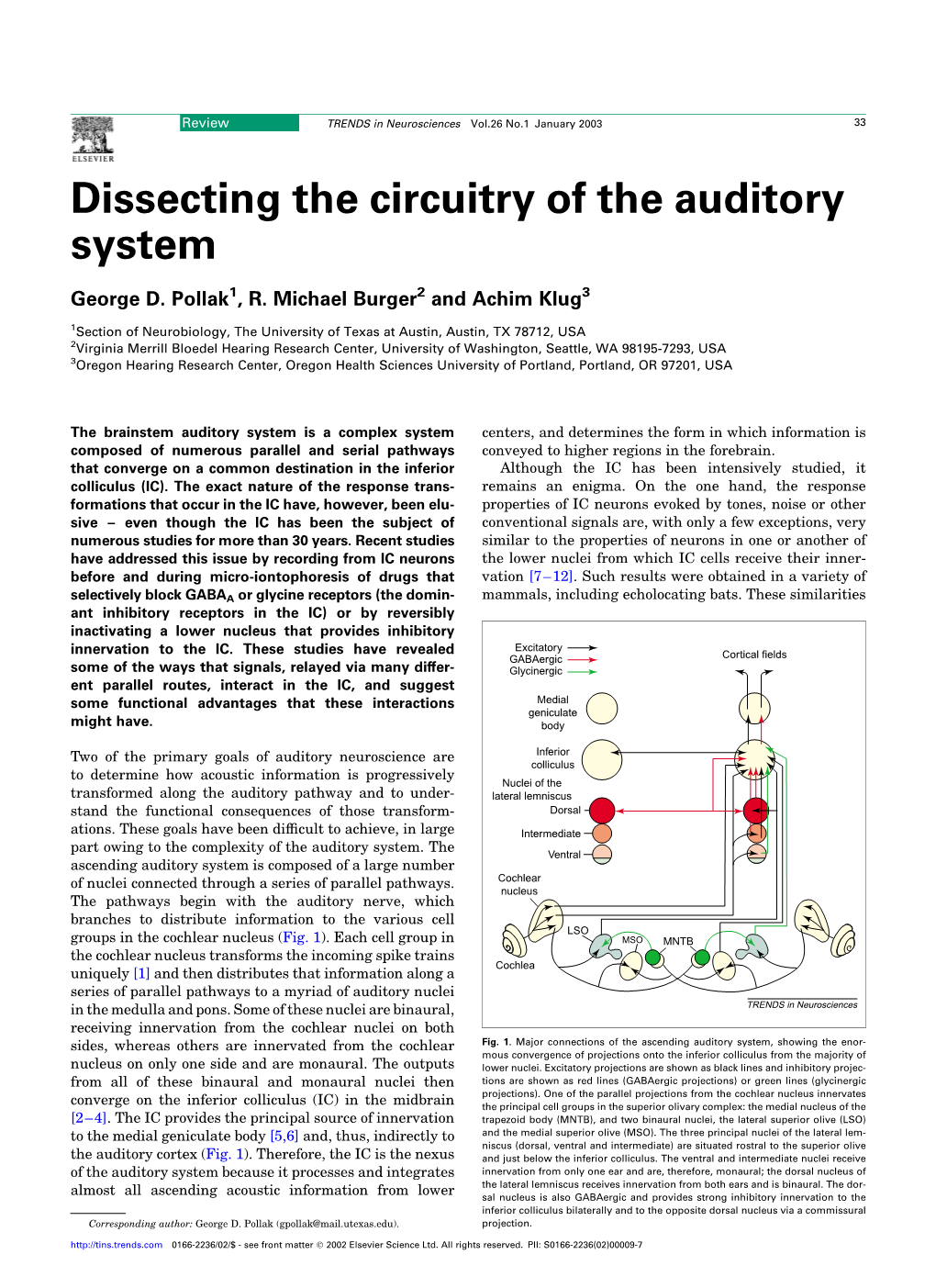
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Projections of Physiologically Defined Subdivisions of the Inferior Colliculus in the Mustached Bat: Targets in the Medial Geniculate Body and Extrathalamic Nuclei
THE JOURNAL OF COMPARATIVE NEUROLOGY 346207-236 (1994) Projections of Physiologically Defined Subdivisions of the Inferior Colliculus in the Mustached Bat: Targets in the Medial Geniculate Body and Extrathalamic Nuclei JEFFREY J. WENSTRUP, DAVID T. LARUE, AND JEFFERY A. WINER Department of Neurobiology, Northeastern Ohio Universities College of Medicine, Rootstown, Ohio 44272-0095 (J.J.W.) and Division of Neurobiology, Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, California 94720-3200 (J.J.W.,D.T.L., J.A.W.) ABSTRACT This study examined the output of the central nucleus of the inferior colliculus to the medial geniculate body and other parts of the nervous system in the mustached bat (Pteronotus parnellii). Small deposits of anterograde tracers (horseradish peroxidase, [3Hlleucine, Phaseo- lus uulgaris leucoagglutinin, wheat germ agglutinin conjugated to horseradish peroxidase, or biocytin) were made at physiologically defined sites in the central nucleus representing major components of the bat’s echolocation signal. The topography, frequency specificity, and axonal morphology of these outputs were studied. The medial geniculate body was a major target of inferior collicular neurons, with three distinct input patterns. The projection to the ventral division was tonotopically organized, but had a relatively sparse contribution from neurons representing frequency modulated compo- nents of the biosonar pulse. The second input was to the rostral medial geniculate body, in which projections from inferior collicular neurons representing constant frequency sonar components were separated from those representing frequency modulated components. A third input was to the suprageniculate nucleus, which received strong, topographically arranged projections. Inputs to the dorsal nucleus and medial division were also observed. -

THE CEREBELLAR VERMIS ROBUSTLY MODULATES NEURAL ACTIVITY in the INFERIOR COLLICULUS by Richard Jiang Sima
THE CEREBELLAR VERMIS ROBUSTLY MODULATES NEURAL ACTIVITY IN THE INFERIOR COLLICULUS by Richard Jiang Sima A dissertation submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland May 2019 © 2019 Richard Jiang Sima All Rights Reserved. Abstract of the Dissertation To survive in an ever-changing world, animals need to rapidly detect and appropriately respond to external stimuli. In the auditory system, the inferior colliculus (IC) is well-positioned as an obligatory auditory hub involved with making acousticomotor responses to initiate these behavioral responses to auditory stimuli. However, it is not well understood how the relevant contextual information needed to detect and respond appropriately to stimuli is conveyed. In this thesis, I investigate the cerebellar vermis as one possible source of this contextual information. My experiments revealed that optogenetic stimulation of the cerebellar vermis robustly modulates the majority of neurons throughout IC in awake mice head-fixed on a treadmill. Because I was to monitor the movement of the treadmill, I also found that vermis stimulation produces a similar behavioral response as auditory stimuli, and that vermis evoked activity in the IC is in part related to this motor response. Furthermore, my results show that animal running modulates IC activity by reducing auditory and vermis responses, while increasing spontaneous activity. Thesis Committee: Professor Sascha du Lac, Thesis Advisor Professor David J. Linden, Chair Professor Jeremiah Y. Cohen, Thesis Reader Professor Amanda M. Lauer Professor Elisabeth B. Glowatzki ii This thesis is dedicated to my mom and dad, Drs. Qing Jiang and Hong Sima, and to my sister, Renee Sima. -

Tonotopic Organization in the Depth of Human Inferior Colliculus
ORIGINAL RESEARCH ARTICLE published: 19 September 2013 HUMAN NEUROSCIENCE doi: 10.3389/fnhum.2013.00586 Tonotopic organization in the depth of human inferior colliculus David Ress 1* and Bharath Chandrasekaran2 1 Neuroscience Department, Center for Perceptual Systems, Imaging Research Center, Institute for Neuroscience, The University of Texas at Austin, Austin TX, USA 2 Department of Communication Sciences and Disorders, Center for Perceptual Systems, Institute for Neuroscience, The University of Texas at Austin, Austin, TX, USA Edited by: Experiments in animal models indicate that inferior colliculus (IC), the primary auditory mid- Florian Beissner, Martinos Center for brain structure, represents sound frequency in a particular spatial organization, a tonotopy, Biomedical Imaging, USA that proceeds from dorsal and superficial to ventral and deeper tissue. Experiments are Reviewed by: Elia Formisano, Maastricht University, presented that use high-resolution, sparse-sampling functional magnetic resonance imag- Netherlands ing (fMRI) at 3T to determine if tonotopic gradients can be reliably measured in human Marc Schönwiesner, University of IC using high-resolution fMRI. Stimuli were sequences of bandpass-filtered noise with dif- Montreal, Canada ferent center frequencies, presented sequentially while fMRI data were collected. Four *Correspondence: subjects performed an adaptive frequency-discrimination task throughout the experiment. David Ress, Neuroscience Results show statistically significant tonotopic gradients within both ICs of all subjects. Department, Baylor College of Medicine, 1 Baylor Plaza, S104N MS Frequency gradients as a function of depth were measured using surface-based analysis BCM240, Houston, TX 77030, USA methods that make virtual penetrations into the IC tissue. This organization was evident e-mail: [email protected] over substantial portions of the IC, at locations that are consistent with the expected loca- tion of the central nucleus of IC.The results confirm a laminar tonotopy in the human IC at 3T, but with a heterogeneous, patchy character. -

The Vestibular-Auditory Interaction for Auditory Brainstem Response to Low Frequencies
Hindawi Publishing Corporation ISRN Otolaryngology Volume 2014, Article ID 103598, 5 pages http://dx.doi.org/10.1155/2014/103598 Clinical Study The Vestibular-Auditory Interaction for Auditory Brainstem Response to Low Frequencies Seyede Faranak Emami and Nasrin Gohari Department of Audiology, Faculty of Rehabilitation, Hamadan University of Medical Sciences and Health Services, Hamadan 16657-696, Iran Correspondence should be addressed to Seyede Faranak Emami; faranak [email protected] Received 5 February 2014; Accepted 27 February 2014; Published 31 March 2014 Academic Editors: A. Horii and J. Meinzen-Derr Copyright © 2014 S. F. Emami and N. Gohari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Since saccular projection is sound sensitive, the objective is to investigate the possibility that the saccular projections may contribute to auditory brainstem response to 500 HZ tone burst (ABR500 HZ). During the case-control research, twenty healthy controls compared to forty selected case groups as having chronic and resistant BPPV were evaluated in the audiology department of Hamadan University of Medical Sciences (Hamadan, Iran). Assessment is comprised of audiologic examinations, cervical vestibular evoked myogenic potentials (cVEMPs), and ABR500 HZ. We found that forty affected ears of BPPV patients with decreased vestibular excitability as detected by abnormal cVEMPs had abnormal results in ABR500 HZ, whereas unaffected ears presented normal findings. Multiple comparisons of mean p13, n23 latencies, and peak-to-peak amplitudes between three groups (affected, unaffected, and healthy ears) were significant. In conclusion, the saccular nerves can be projective to auditory bundles and interact with auditory brainstem response to low frequencies. -

Pax2-Islet1 Transgenic Mice Are Hyperactive and Have Altered Cerebellar Foliation
Mol Neurobiol (2017) 54:1352–1368 DOI 10.1007/s12035-016-9716-6 Pax2-Islet1 Transgenic Mice Are Hyperactive and Have Altered Cerebellar Foliation Romana Bohuslavova1 & Nicole Dodd1 & Iva Macova1 & Tetyana Chumak 2 & Martin Horak3 & Josef Syka2 & Bernd Fritzsch 4 & Gabriela Pavlinkova1 Received: 7 September 2015 /Accepted: 12 January 2016 /Published online: 3 February 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract The programming of cell fate by transcription fac- competitive channel blocker for the γ-aminobutyric acid tors requires precise regulation of their time and level of ex- (GABA) receptor chloride channels. This suggests that the pression. The LIM-homeodomain transcription factor Islet1 overexpression of Isl1 significantly affects the functions of (Isl1) is involved in cell-fate specification of motor neurons, GABAergic neurons. We demonstrate that the overexpression and it may play a similar role in the inner ear. In order to study of Isl1 affects the development and function of the cerebello- its role in the regulation of vestibulo-motor development, we vestibular system, resulting in hyperactivity. investigated a transgenic mouse expressing Isl1 under the − Pax2 promoter control (Tg+/ ). The transgenic mice show Keywords Islet1 transcription factor . Vestibular system . altered level, time, and place of expression of Isl1 but are Cerebellum . Foliation defects . Hyperactivity . GABA − viable. However, Tg+/ mice exhibit hyperactivity, including signaling . Transgenic mouse . Purkinje cells . Calcium circling behavior, and progressive age-related decline in hear- homeostasis . Age-related deterioration of Purkinje cells . ing, which has been reported previously. Here, we describe the Attention deficit hyperactivity disorder molecular and morphological changes in the cerebellum and vestibular system that may cause the hyperactivity of Tg+/− mice. -

The Vestibulo- Cochlear Nerve)
Cranial Nerve VIII (The Vestibulo- Cochlear Nerve) Lecture (11) ▪ Important ▪ Doctors Notes Please check our Editing File ▪ Notes/Extra explanation ه هذا العمل مب ين بشكل أسا يس عىل عمل دفعة 436 مع المراجعة { َوَم نْ يَ َت َو َ ّكْ عَ َلْ ا َّْلل فَهُ َوْ َحْ سْ ُ ُُْ} والتدقيق وإضافة المﻻحظات وﻻ يغ ين عن المصدر اﻷسا يس للمذاكرة ▪ Objectives At the end of the lecture, students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus Brain – Ventral Surface (cerebello-pontine angle) Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts attach to the ventral surface* of brain stem through the pontomedullary sulcus at the junction of the medulla & pons (cerebellopontine angle)* (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. -

Gabaergic Feedforward Projections from the Inferior Colliculus to the Medial Geniculate Body JEFFERY A
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 8005-8010, July 1996 Neurobiology GABAergic feedforward projections from the inferior colliculus to the medial geniculate body JEFFERY A. WINER*t, RICHARD L. SAINT MARIEt, DAVID T. LARUE*, AND DOUGLAS L. OLIVER§ *Division of Neurobiology, Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, CA 94720-3200; tDepartment of Neuroanatomy, The House Ear Institute, 2100 West Third Street, Los Angeles, CA 90057; and §Department of Anatomy, The University of Connecticut Health Center, Farmington, CT 06030-3405 Communicated by Carla J. Shatz, University of California, Berkeley, CA, February 21, 1996 (received for review November 27, 1995) ABSTRACT A novel and robust projection from y-ami- with isoflurane at a level that suppressed all nociceptive nobutyric acid-containing (GABAergic) inferior colliculus reflexes. All experimental procedures followed the applicable neurons to the medial geniculate body (MGB) was discovered and approved institutional animal care and use protocols. Four in the cat using axoplasmic transport methods combined with unilateral penetrations were made using stereotaxic coordi- immunocytochemistry. This input travels with the classical nates to target specific nuclei in various subdivisions. A total inferior colliculus projection to the MGB, and it is a direct of seven, 50-nl deposits were made at 500-,um intervals along ascending GABAergic pathway to the sensory thalamus that a track with a glass micropipet; the net volume was -1.5 ,lI. may be inhibitory. This bilateral projection constitutes 10- The animal was reanesthetized 3 days later and perfused 30% of the neurons in the auditory tectothalamic system. with PBS followed by 2% paraformaldehyde and 3% glutar- Studies by others have shown that comparable input to the aldehyde in 0.12 M PB. -

The Connections of the Inferior Colliculus and the Organization Of
THE JOURNAL OF COMPARATIVE NEUROLOGY 201:25-49 (1981) The Connections of the Inferior Colliculus and the Organization of the Brainstem Auditory System in the Greater Horseshoe Bat (Rhinolophus ferrumequinum) HERMANN SCHWEIZER Arbeitskreis Neuro- und Rezeptorphyszologte, Fachbereich Biologie (Zoologre), J W Goethe Unzuersttaet, D 6000 FrankfurtlM Federal Republic of Germany ABSTRACT The connections of the inferior colliculus, the mammalian mid- brain auditory center, were determined in the greater horseshoe bat (Rhinolophus ferrumequinum), using the horseradish peroxidase method. In order to localize the auditory centers of this bat, brains were investigated with the aid of cell and fiber-stained material. The results show that most auditory centers are highly developed in this echo- locating bat. However, the organization of the central auditory system does not generally differ from the mammalian scheme. This holds also for the organization of the superior olivary complex where a well-developed medial superior olivary nucleus was found. In addition to the ventral and dorsal nuclei of the lateral lemniscus a third well-developed nucleus has been defined which projects ipsi- laterally to the inferior colliculus and which was called the intermediate nucleus of the lateral leminiscus. All nuclei of the central auditory pathway project ipsi-, contra-, or bilaterally to the central nucleus of the inferior colliculus with the exception of the medial nucleus of the trapezoid body and the medial geniculate body. The tonotopic organization of these projections and their possible functions are discussed in context with neurophysiological investigations. Insectivorous bats are mammals that hunt are the higher auditory centers, the medial at night with the help of an active sonar sys- geniculate body, and the auditory cortex? tem.