Generated by SRI International Pathway Tools Version 25.0, Authors S
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Metabolic Engineering of Microorganisms for the Overproduction of Fatty Acids Ting Wei Tee Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2013 Metabolic engineering of microorganisms for the overproduction of fatty acids Ting Wei Tee Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Chemical Engineering Commons Recommended Citation Tee, Ting Wei, "Metabolic engineering of microorganisms for the overproduction of fatty acids" (2013). Graduate Theses and Dissertations. 13516. https://lib.dr.iastate.edu/etd/13516 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Metabolic engineering of microorganisms for the overproduction of fatty acids by Ting Wei Tee A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of Doctor of Philosophy Major: Chemical Engineering Program of Study Committee: Jacqueline V. Shanks, Major Professor Laura R. Jarboe R. Dennis Vigil David J. Oliver Marna D. Nelson Iowa State University Ames, Iowa 2013 Copyright © Ting Wei Tee, 2013. All rights reserved. ii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ................................................................................................ v ABSTRACT ....................................................................................................................... -

Demethylation and Degradation of Phenylmethylethers by the Sulfide-Methylating Homoacetogenic Bacterium Strain TMBS 4
Arch Microbiol (1993) 159:308-315 Archives of Hicrobiology 9Sprlnger-Verlag 1993 Demethylation and degradation of phenylmethylethers by the sulfide-methylating homoacetogenic bacterium strain TMBS 4 Jan-Ulrich Kreft, Bernhard Schink Fakult/it ffir Biologie, Universit/it Konstanz, Postfach 5560, W-7750 Konstanz, Germany Received: 5 July 1992/Accepted: 26 October 1992 Abstract. Biochemical studies on anaerobic phenylme- Key words: Methyl transfer - Demethylation - Me- thylether cleavage by homoacetogenic bacteria have been thoxylated aromatic compounds - Ether cleavage - hampered so far by the complexity of the reaction chain Corrinoids - Homoacetogenic bacteria - Phloroglucin- involving methyl transfer to acetyl-CoA synthase and ol pathway - Dimethylsulfide subsequent methyl group carbonylation to acetyl-CoA. Strain TMBS 4 differs from other demethylating homo- acetogenic bacteria in using sulfide as a methyl accep- tor, thereby forming methanethiol and dimethylsulfide. Growing and resting cells of strain TMBS 4 used alternati- Aerobic degradation of ether compounds is a well-known tively CO 2 as a precursor of the methyl acceptor CO for process (Axelrod 1956). Hydroxylation of a carbon atom homoacetogenic acetate formation. Demethylation was vicinal to the ether bridge by a monooxygenase reaction inhibited by propyl iodide and reactivated by light, transforms the stable ether bond into a readily decom- indicating involvement of a corrinoid-dependent methyl- posing hemiacetal structure (Bernhardt et al. 1970). An- transferase. Strain TMBS 4 contained ca. 750 nmol g dry aerobic cleavage of ether linkages was first demonstrated mass - 1 of a corrinoid tentatively identified as 5-hydroxy- in methanogenic enrichment cultures (Healy and Young benzimidazolyl cobamide. A photometric assay for meas- 1979), and pure cultures of homoacetogenic bacteria uring the demethylation activity in cell extracts was demethylating methoxylated aromatic compounds were developed based on the formation of a yellow complex isolated later (Bache and Pfennig 1981). -

JOURNAL of BACTERIOLOGY VOLUME 169 DECEMBER 1987 NUMBER 12 Samuel Kaplan, Editor in Chief (1992) Kenneth N
JOURNAL OF BACTERIOLOGY VOLUME 169 DECEMBER 1987 NUMBER 12 Samuel Kaplan, Editor in Chief (1992) Kenneth N. Timmis, Editor (1992) University of Illinois, Urbana Richard M. Losick, Editor (1988) Centre Medical Universitaire, James D. Friesen, Editor (1992) Harvard University, Cambridge, Mass. Geneva, Switzerland University of Toronto, L. Nicholas Ornston, Editor (1992) Graham C. Walker, Editor (1990) Toronto, Canada Yale University, New Haven, Conn. Massachusetts Institute of Stanley C. Holt, Editor (1987) Robert H. Rownd, Editor (1990) Technology, Cambridge, Mass. The University of Texas Health Northwestern Medical School, Robert A. Weisberg, Editor (1990) Science Center, San Antonio Chicago, Ill. National Institute of Child June J. Lascelles, Editor (1989) Health and Human University of California, Los Angeles Development, Bethesda, Md. EDITORIAL BOARD David Apirion (1988) James G. Ferry (1989) Eva R. Kashket (1987) Palmer Rogers (1987) Stuart J. Austin (1987) David Figurski (1987) David E. Kennell (1988) Barry P. Rosen (1989) Frederick M. Ausubel (1989) Timothy J. Foster (1989) Wil N. Konings (1987) Lucia B. Rothman-Denes (1989) Barbara Bachmann (1987) Robert T. Fraley (1988) Jordan Konisky (1987) Rudiger Schmitt (1989) Manfred E. Bayer (1988) David I. Friedman (1989) Dennis J. Kopecko (1987) June R. Scott (1987) Margret H. Bayer (1989) Masamitsu Futai (1988) Viji Krishnapillai (1988) Jane K. Setlow (1987) Claire M. Berg (1989) Robert Gennis (1988) Terry Krulwich (1987) Peter Setlow (1987) Helmut Bertrand (1988) Jane Gibson (1988) Lasse Lindahl (1987) James A. Shapiro (1988) Terry J. Beveridge (1988) Robert D. Goldman (1988) Jack London (1987) Louis A. Sherman (1988) Donald A. Bryant (1988) Susan Gottesman (1989) Sharon Long (1989) Howard A. -
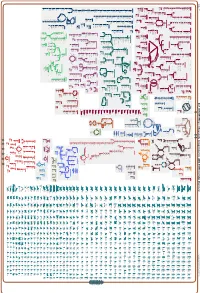
Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_003855395Cyc: Shewanella livingstonensis LMG 19866 Cellular Overview Connections between pathways are omitted for legibility. -

Antiviral Compounds Antivirale Verbindungen Composes Antiviraux
(19) TZZ_Z _T (11) EP 1 778 702 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07F 9/141 (2006.01) C07D 207/08 (2006.01) 13.07.2011 Bulletin 2011/28 A61K 31/662 (2006.01) A61P 31/12 (2006.01) (21) Application number: 05791144.8 (86) International application number: PCT/US2005/025503 (22) Date of filing: 18.07.2005 (87) International publication number: WO 2006/020276 (23.02.2006 Gazette 2006/08) (54) ANTIVIRAL COMPOUNDS ANTIVIRALE VERBINDUNGEN COMPOSES ANTIVIRAUX (84) Designated Contracting States: (74) Representative: Reitstötter - Kinzebach AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Patentanwälte HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI Sternwartstrasse 4 SK TR 81679 München (DE) Designated Extension States: AL BA HR MK YU (56) References cited: EP-A- 1 337 550 WO-A-00/09543 (30) Priority: 16.07.2004 US 588633 P WO-A-00/59929 WO-A-98/17679 27.07.2004 US 591635 P WO-A-99/07733 WO-A-02/060926 WO-A-03/053349 WO-A-03/064416 (43) Date of publication of application: WO-A-03/064455 WO-A-03/064456 02.05.2007 Bulletin 2007/18 WO-A-03/066103 WO-A-03/099274 WO-A-03/099316 US-A1- 2003 186 895 (60) Divisional application: US-A1- 2003 224 977 US-A1- 2004 048 802 10178084.9 / 2 316 839 US-B1- 6 608 027 (73) Proprietor: GILEAD SCIENCES, INC. -

Evidence of Two Oxidative Reaction Steps Initiating Anaerobic Degradation of Resorcinol (1,3-Dihydroxybenzene) by the Denitrifying Bacterium Azoarcus Anaerobius
JOURNAL OF BACTERIOLOGY, July 1998, p. 3644–3649 Vol. 180, No. 14 0021-9193/98/$04.0010 Copyright © 1998, American Society for Microbiology. All Rights Reserved. Evidence of Two Oxidative Reaction Steps Initiating Anaerobic Degradation of Resorcinol (1,3-Dihydroxybenzene) by the Denitrifying Bacterium Azoarcus anaerobius BODO PHILIPP* AND BERNHARD SCHINK Fakulta¨t fu¨r Biologie, Mikrobielle O¨ kologie, Universita¨t Konstanz, D-78457 Konstanz, Germany Received 21 January 1998/Accepted 11 May 1998 The denitrifying bacterium Azoarcus anaerobius LuFRes1 grows anaerobically with resorcinol (1,3-dihydroxy- benzene) as the sole source of carbon and energy. The anaerobic degradation of this compound was investi- gated in cell extracts. Resorcinol reductase, the key enzyme for resorcinol catabolism in fermenting bacteria, was not present in this organism. Instead, resorcinol was hydroxylated to hydroxyhydroquinone (HHQ; 1,2,4-trihydroxybenzene) with nitrate or K3Fe(CN)6 as the electron acceptor. HHQ was further oxidized with nitrate to 2-hydroxy-1,4-benzoquinone as identified by high-pressure liquid chromatography, UV/visible light spectroscopy, and mass spectroscopy. Average specific activities were 60 mU mg of protein21 for resorcinol hydroxylation and 150 mU mg of protein21 for HHQ dehydrogenation. Both activities were found nearly exclusively in the membrane fraction and were only barely detectable in extracts of cells grown with benzoate, indicating that both reactions were specific for resorcinol degradation. These findings suggest a new strategy of anaerobic degradation of aromatic compounds involving oxidative steps for destabilization of the aromatic ring, different from the reductive dearomatization mechanisms described so far. The biochemistry of anaerobic degradation of aromatic In this work, we reexamined resorcinol degradation by A. -

Purification and Characterization of the Higher Plant Enzyme L-Canaline Reductase (L-Canavanine Catabolism/Plant Nitrogen Metabolism/Leguminosae) GERALD A
Proc. Natd. Acad. Sci. USA Vol. 89, pp. 1780-1784, March 1992 Biochemistry Purification and characterization of the higher plant enzyme L-canaline reductase (L-canavanine catabolism/plant nitrogen metabolism/Leguminosae) GERALD A. ROSENTHAL T. H. Morgan School of Biological Sciences, University of Kentucky, Lexington, KY 40506 Communicated by John S. Boyer, December 3, 1991 (receivedfor review April 20, 1991) ABSTRACT A newly discovered enzyme, L-canaline re- oglutaric acid to generate stoichiometrically a canaline-2- ductase (NADPH:L-canaline oxidoreductase, EC 1.6.6.-), has oxoglutaric acid oxime (5). Canaline also reacts readily with been isolated and purified from 10-day-old leaves of the jack the pyridoxal phosphate moiety of vitamin B6-containing bean Canavalia ensiformis (Leguminosae). This higher plant is enzymes to form a stable, covalently linked complex (6, 7). representative of a large number of legumes that synthesize In vitro analysis of canaline interaction with homogeneous L-canavanine, an important nitrogen-storing nonprotein amino ornithine aminotransferase (ornithine-oxo-acid aminotrans- acid. Canavanine-storing legumes contain arginase, which ferase; L-ornithine:2-oxo-acid aminotransferase, EC hydrolyzes L-canavanine to form the toxic metabolite L-cana- 2.6.1.13) reveals its marked ability to form an oxime complex line. Canaline reductase, having a mass of =167 kDa and with and thereby inhibit this pyridoxal phosphate-dependent composed of 82-kDa dimers, catalyzes a NADPH-dependent enzyme (8). As little as 10 nM canaline causes a significant reductive cleavage of L-canaline to L-homoserine and ammo- reduction in ornithine aminotransferase activity (9). nia. This is the only enzyme known to use reduced NADP to Canavanine-storing legumes can accumulate high levels of cleave an O-N bond. -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -

(12) United States Patent (10) Patent No.: US 7,776,844 B2 Yu Et Al
USOO777684.4B2 (12) United States Patent (10) Patent No.: US 7,776,844 B2 Yu et al. (45) Date of Patent: Aug. 17, 2010 (54) N-(PHOSPHONOALKYL)-AMINO ACIDS, 7,429,575 B2 9/2008 Yu et al. DERVATIVES THEREOF AND 2005, 016491.6 A1 7/2005 Leadbetter et al. COMPOSITIONS AND METHODS OF USE OTHER PUBLICATIONS (76) Inventors: Ruey J. Yu, 655 Stump Rd., Chalfont, Struninet al., 1989, CAS. 111:233525.* PA (US)18914; Eugene J. Van Scott, 3 Shi et al., 2004, CAS;142. 231739.* Hidden La., Abington, PA (US) 19001 Li et al., 2005, CAS; 143: 158351.* Sandeman et al., 2002, CAS: 137:274431.* (*) Notice: Subject to any disclaimer, the term of this Single co's i. 164: patent is extended or adjusted under 35 Stahl e et et al., al., 1995, CAS:si. 124:563.10.* U.S.C. 154(b) by 0 days. Jezowska-Bojczuk et al., 1994, CAS: 120:28.1638.* Strumin et al., 1989, CAS: 111:194894.* (21) Appl. No.: 12/428,906 Balthazor et al., 1987, CAS: 106:50457.* The Merck Index. "An Encyclopedia of Chemicals, Drugs, and (22) Filed: Apr. 23, 2009 Biologicals.” (2001) p. 1768, (2 pgs.), 13th Edition, O'Neil et al. (Ed.), Merck & Co., Inc., Whitehouse Station, N.J. (65) Prior Publication Data Wester et al., 1991, CAS: 114:242443. US 2009/0208499 A1 Aug. 20, 2009 * cited by examiner Related U.S. Application Data Primary Examiner Rei-tsang Shiao - - - (74) Attorney, Agent, or Firm Panitch Schwarze Belisario & (62) Division of application No. 12/194203, filed on Aug. -

| Mo Naman Attituunika Mitatti
|MO NAMAN ATTITUUNIKAUS009962450B2 MITATTI (12 ) United States Patent (10 ) Patent No. : US 9 , 962 , 450 B2 Kraynov et al. ( 45) Date of Patent : May 8 , 2018 ( 54 ) METHOD OF TREATING HEART FAILURE 4 ,659 ,839 A 4 / 1987 Nicolotti et al . WITH MODIFIED RELAXIN POLYPEPTIDES 4 ,670 ,417 A 6 / 1987 Iwasaki et al. 4 ,671 , 958 A 6 / 1987 Rodwell et al. 4 ,680 , 338 A 7 / 1987 Sundoro ( 71) Applicant : AMBRX , INC . , La Jolla , CA (US ) 4 ,689 ,406 A 8 / 1987 Banks et al . 4 ,699 ,784 A 10 / 1987 Shih et al . ( 72 ) Inventors : Vadim Kraynov , San Diego , CA (US ) ; 4 ,738 , 921 A 4 / 1988 Belagaje et al. Nick Knudsen , San Diego , CA (US ) ; 4 ,755 ,465 A 7 / 1988 Gray et al. 4 ,837 , 148 A 6 / 1989 Cregg Amha Hewet, Chula Vista , CA (US ) ; 4 ,859 ,600 A 8 / 1989 Gray et al . Kristine De Dios, San Diego , CA (US ) ; 4 , 876 , 197 A 10 / 1989 Burke et al. Jason Pinkstaff , Encinitas, CA (US ) ; 4 , 880 ,734 A 11/ 1989 Burke et al . Lorraine Sullivan , San Diego , CA 4 , 902 ,502 A 2 / 1990 Nitecki et al . 4 , 904 , 584 A 2 / 1990 Shaw ( US ) 4 ,929 , 555 A 5 / 1990 Cregg et al. 5 ,021 , 234 A 6 / 1991 Ehrenfeld (73 ) Assignee : AMBRX , INC. , La Jolla , CA (US ) 5 ,089 ,398 A 2 / 1992 Rosenberg et al . 5 , 122 ,614 A 6 / 1992 Zalipsky ( * ) Notice : Subject to any disclaimer , the term of this 5 , 145 , 962 A 9 / 1992 Hudson et al. -

Zone Finhibton Inmillimeters
US006191168B1 (12) United States Patent (10) Patent N0.: US 6,191,168 B1 Rubenstein (45) Date of Patent: Feb. 20, 2001 (54) METHODS FOR THE USE OF NONPROTEIN CA 126: 268319, Breton et al, 1997.* AMINO ACIDS AS THERAPEUTIC AGENTS Bell. (1958). “Canavanine and Related Compounds in Legu minosae,” Biochem J. 70:617—619. (75) Inventor: Edward Rubenstein, 5 Waverly Pl., Bisby et al. (1994). Phytochemical Dictionary of the Legu Hillsborough, CA (US) 94010 minose, vol. 1—2. (Title page and table of contents only). Butler et al. (1967). “Uptake and Metabolism of Inorganic (73) Assignee: Edward Rubenstein, Hillsborough, CA Forms of Selenium—75 by Spiroa'ela Oligorrhiza, ” Aust. J. (Us) Biol. Sci. 20:77—86. Conn. (1981). “Secondary Plant Products” The Biochemistry (*) Notice: Under 35 U.S.C. 154(b), the term of this of Plants vol. 7(Title page and table of contents only). patent shall be extended for 0 days. CoWie et al. (1957). “Biosynthesis by Escherichia Coli of Active Altered Proteins Containing Selenium Instead of (21) Appl. No.: 09/324,181 Sulfur,” Biochem et Biophysica Acta. 26:252—261. Gennaro ed. (1995). Remington." The Science and Practice Filed: Jun. 1, 1999 (22) The Science and of Pharmacy (Title page and table of Related US. Application Data contents only). (60) Provisional application No. 60/087,746, ?led on Jun. 2, (List continued on neXt page.) 1998. Primary Examiner—Rebecca Cook (51) Int. Cl.7 ................................................. .. A61K 31/195 (74) Attorney, Agent, or Firm—Morrison & Foerster LLP (52) US. Cl. ........................ .. 514/561; 514/210; 514/274; 514/419; 514/423; 514/315; 514/354; 514/538; (57) ABSTRACT 514/562; 514/563; 514/564; 514/565 Provided are compositions comprising nonprotein amino (58) Field of Search .................................. -

Download Product Insert (PDF)
PRODUCT INFORMATION L-Canaline Item No. 9002357 CAS Registry No.: 496-93-5 Formal Name: O-amino-L-homoserine O MF: C H N O 4 10 2 3 O FW: 134.1 HO NH2 Purity: ≥95% NH Stability: ≥2 years at -20°C 2 Supplied as: A crystalline solid Laboratory Procedures For long term storage, we suggest that L-canaline be stored as supplied at -20°C. It should be stable for at least two years. L-Canaline is supplied as a crystalline solid. A stock solution may be made by dissolving the L-canaline in the solvent of choice. L-Canaline is soluble in organic solvents such as DMSO which should be purged with an inert gas. The solubility of L-canaline in this solvent is approximately 1 mg/ml. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of L-canaline can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of L-canaline in PBS, pH 7.2, is approximately 10 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description L-Canaline is an aminooxy analog of ornithine that irreversibly inhibits aminotransferases (transaminases), 1-3 including ornithine aminotransferase (Ki = 2 µM). It forms oximes with α-keto acids and aldehydes, most notably with pyridoxal phosphate, an essential cofactor of aminotransferases.3 L-Canaline is naturally found in plants, including legumes, and is involved in the metabolism of L-canavanine, an aminooxy analog of arginine.4 It is cytotoxic to a range of organisms, including bacteria, insects, and parasites.2,4,5 References 1.