Prediction of Disordered Regions and Their Roles in the Anti-Pathogenic and Immunomodulatory Functions of Butyrophilins
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Genome-Wide Approach to Identify Risk Factors for Therapy-Related Myeloid Leukemia
Leukemia (2006) 20, 239–246 & 2006 Nature Publishing Group All rights reserved 0887-6924/06 $30.00 www.nature.com/leu ORIGINAL ARTICLE Genome-wide approach to identify risk factors for therapy-related myeloid leukemia A Bogni1, C Cheng2, W Liu2, W Yang1, J Pfeffer1, S Mukatira3, D French1, JR Downing4, C-H Pui4,5,6 and MV Relling1,6 1Department of Pharmaceutical Sciences, The University of Tennessee, Memphis, TN, USA; 2Department of Biostatistics, The University of Tennessee, Memphis, TN, USA; 3Hartwell Center, The University of Tennessee, Memphis, TN, USA; 4Department of Pathology, The University of Tennessee, Memphis, TN, USA; 5Department of Hematology/Oncology St Jude Children’s Research Hospital, The University of Tennessee, Memphis, TN, USA; and 6Colleges of Medicine and Pharmacy, The University of Tennessee, Memphis, TN, USA Using a target gene approach, only a few host genetic risk therapy increases, the importance of identifying host factors for factors for treatment-related myeloid leukemia (t-ML) have been secondary neoplasms increases. defined. Gene expression microarrays allow for a more 4 genome-wide approach to assess possible genetic risk factors Because DNA microarrays interrogate multiple ( 10 000) for t-ML. We assessed gene expression profiles (n ¼ 12 625 genes in one experiment, they allow for a ‘genome-wide’ probe sets) in diagnostic acute lymphoblastic leukemic cells assessment of genes that may predispose to leukemogenesis. from 228 children treated on protocols that included leukemo- DNA microarray analysis of gene expression has been used to genic agents such as etoposide, 13 of whom developed t-ML. identify distinct expression profiles that are characteristic of Expression of 68 probes, corresponding to 63 genes, was different leukemia subtypes.13,14 Studies using this method have significantly related to risk of t-ML. -

Modulated in Intestinal Inflammation A
BTNL2, a Butyrophilin/B7-Like Molecule, Is a Negative Costimulatory Molecule Modulated in Intestinal Inflammation This information is current as Heather A. Arnett, Sabine S. Escobar, Eva Gonzalez-Suarez, of September 28, 2021. Alison L. Budelsky, Lori A. Steffen, Norman Boiani, Ming Zhang, Gerald Siu, Avery W. Brewer and Joanne L. Viney J Immunol 2007; 178:1523-1533; ; doi: 10.4049/jimmunol.178.3.1523 http://www.jimmunol.org/content/178/3/1523 Downloaded from References This article cites 40 articles, 12 of which you can access for free at: http://www.jimmunol.org/content/178/3/1523.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2007 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology BTNL2, a Butyrophilin/B7-Like Molecule, Is a Negative Costimulatory Molecule Modulated in Intestinal Inflammation Heather A. Arnett,1* Sabine S. -
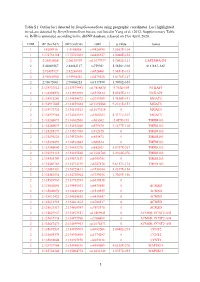
1 Table S1. Outlier Loci Detected by Deepgenomescan Using
Table S1. Outlier loci detected by DeepGenomeScan using geographic coordinates. Loci highlighted in red are detected by DeepGenomeScan but are not listed in Yang et al. (2012; Supplementary Table 4). RsID is annotated according to the dbSNP database released on 21st April, 2020. CHR BP (Grch37) BP (Grch38) rsID p.value Genes 1 1:4208918 1:4148858 rs9426495 3.8582E-104 1 1:175738168 1:175769032 rs6425357 2.0088E-105 2 2:20310668 2:20110907 rs11679737 1.2002E-111 LAPTM4A-DT 2 2:40289267 2:40062127 rs759361 5.5658E-104 SLC8A1-AS1 2 2:82495127 2:82268003 rs6726401 3.9451E-103 2 2:96660300 2:95994552 rs2579520 1.8176E-127 2 2:96672001 2:96006253 rs1917890 3.7698E-104 2 2:134333012 2:133575441 rs17816830 9.702E-105 NCKAP5 2 2:134350570 2:133592999 rs6715224 5.8745E-111 NCKAP5 2 2:134912243 2:134154672 rs2139309 1.7418E-191 MGAT5 2 2:134917005 2:134159434 rs11692586 9.2121E-151 MGAT5 2 2:134972732 2:134215161 rs11679218 0 MGAT5 2 2:134979966 2:134222395 rs1965183 5.3171E-107 MGAT5 2 2:135260071 2:134502500 rs503562 2.057E-153 TMEM163 2 2:135280039 2:134522468 rs579670 3.1477E-118 TMEM163 2 2:135285279 2:134527708 rs512375 0 TMEM163 2 2:135290221 2:134532650 rs655472 0 TMEM163 2 2:135290453 2:134532882 rs666614 0 TMEM163 2 2:135340840 2:134583270 rs842361 5.6717E-237 TMEM163 2 2:135393110 2:134635540 rs11684785 2.2164E-276 TMEM163 2 2:135430709 2:134673139 rs6745983 0 TMEM163 2 2:135469769 2:134712199 rs6747870 9.6117E-114 TMEM163 2 2:135483381 2:134725811 rs3739034 5.0357E-154 2 2:135483534 2:134725964 rs3739036 3.7269E-156 2 2:135539967 2:134782397 -

Role and Regulation of the P53-Homolog P73 in the Transformation of Normal Human Fibroblasts
Role and regulation of the p53-homolog p73 in the transformation of normal human fibroblasts Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Bayerischen Julius-Maximilians-Universität Würzburg vorgelegt von Lars Hofmann aus Aschaffenburg Würzburg 2007 Eingereicht am Mitglieder der Promotionskommission: Vorsitzender: Prof. Dr. Dr. Martin J. Müller Gutachter: Prof. Dr. Michael P. Schön Gutachter : Prof. Dr. Georg Krohne Tag des Promotionskolloquiums: Doktorurkunde ausgehändigt am Erklärung Hiermit erkläre ich, dass ich die vorliegende Arbeit selbständig angefertigt und keine anderen als die angegebenen Hilfsmittel und Quellen verwendet habe. Diese Arbeit wurde weder in gleicher noch in ähnlicher Form in einem anderen Prüfungsverfahren vorgelegt. Ich habe früher, außer den mit dem Zulassungsgesuch urkundlichen Graden, keine weiteren akademischen Grade erworben und zu erwerben gesucht. Würzburg, Lars Hofmann Content SUMMARY ................................................................................................................ IV ZUSAMMENFASSUNG ............................................................................................. V 1. INTRODUCTION ................................................................................................. 1 1.1. Molecular basics of cancer .......................................................................................... 1 1.2. Early research on tumorigenesis ................................................................................. 3 1.3. Developing -

Next Generation Exome Sequencing of Paediatric Inflammatory Bowel Disease Patients Identifies Rare and Novel Variants in Candida
Gut Online First, published on April 28, 2012 as 10.1136/gutjnl-2011-301833 Inflammatory bowel disease ORIGINAL ARTICLE Gut: first published as 10.1136/gutjnl-2011-301833 on 28 April 2012. Downloaded from Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes Katja Christodoulou,1 Anthony E Wiskin,2 Jane Gibson,1 William Tapper,1 Claire Willis,2 Nadeem A Afzal,3 Rosanna Upstill-Goddard,1 John W Holloway,4 Michael A Simpson,5 R Mark Beattie,3 Andrew Collins,1 Sarah Ennis1 < Additional materials are ABSTRACT published online only. To view Background Multiple genes have been implicated by Significance of this study these files please visit the association studies in altering inflammatory bowel journal online (http://gut.bmj. com/content/early/recent). disease (IBD) predisposition. Paediatric patients often What is already known on this subject? manifest more extensive disease and a particularly < For numbered affiliations see Genome-wide association studies have impli- end of article. severe disease course. It is likely that genetic cated numerous candidate genes for inflamma- predisposition plays a more substantial role in this group. tory bowel disease (IBD), but evidence of Correspondence to Objective To identify the spectrum of rare and novel causality for specific variants is largely absent. Dr Sarah Ennis, Genetic variation in known IBD susceptibility genes using exome Furthermore, by design, genome-wide associa- Epidemiology and Genomic sequencing analysis in eight individual cases of childhood Informatics Group, Human tion studies are limited to the study of Genetics, Faculty of Medicine, onset severe disease. -

BTN3A3 (NM 197974) Human Untagged Clone – SC107714
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for SC107714 BTN3A3 (NM_197974) Human Untagged Clone Product data: Product Type: Expression Plasmids Product Name: BTN3A3 (NM_197974) Human Untagged Clone Tag: Tag Free Symbol: BTN3A3 Synonyms: BTF3; BTN3.3 Vector: pCMV6-XL5 E. coli Selection: Ampicillin (100 ug/mL) Cell Selection: None This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 4 BTN3A3 (NM_197974) Human Untagged Clone – SC107714 Fully Sequenced ORF: >OriGene sequence for NM_197974 edited GAATTCGGCACGAGTGCTTTCTTTTTCCTTTCTTCGGAATGAGAGACTCAACCATAATAG AAAGAATGGAGAACTATTAACCACCATTCTTCAGTGGGCTGTGATTTTCAGAGGGGAATA CTAAGAAATGGTTTTCCATACTGGAACCCAAAGGTAAAGACACTCAAGGACAGACATTTT TGGCAGAGCTCAGTTTTCTGTGCTTGGACCCTCTGGGCCCATCCTGGCCATGGTGGGTGA AGACGCTGATCTGCCCTGTCACCTGTTCCCGACCATGAGTGCAGAGACCATGGAGCTGAG GTGGGTGAGTTCCAGCCTAAGGCAGGTGGTGAACGTGTATGCAGATGGAAAGGAAGTGGA AGACAGGCAGAGTGCACCGTATCGAGGGAGAACTTCGATTCTGCGGGATGGCATCACTGC AGGGAAGGCTGCTCTCCGAATACACAACGTCACAGCCTCTGACAGTGGAAAGTACTTGTG TTATTTCCAAGATGGTGACTTCTACGAAAAAGCCCTGGTGGAGCTGAAGGTTGCAGCATT GGGTTCTGATCTTCACATTGAAGTGAAGGGTTATGAGGATGGAGGGATCCATCTGGAGTG CAGGTCCACTGGCTGGTACCCCCAACCCCAAATAAAGTGGAGCGACACCAAGGGAGAGAA CATCCCGGCTGTGGAAGCACCTGTGGTTGCAGATGGAGTGGGCCTGTATGCAGTAGCAGC ATCTGTGATCATGAGAGGCAGCTCTGGTGGGGGTGTATCCTGCATCATCAGAAATTCCCT -

Familial Vs. Sporadic Sarcoidosis: BTNL2 Polymorphisms, Clinical
Familial vs. sporadic sarcoidosis: BTNL2 polymorphisms, clinical presentations, and outcomes in a French cohort Yves Pacheco, Alain Calender, Dominique Israël-Biet, Pascal Roy, Serge Lebecque, Vincent Cottin, Diane Bouvry, Hilario Nunes, Pascal Sève, Laurent Pérard, et al. To cite this version: Yves Pacheco, Alain Calender, Dominique Israël-Biet, Pascal Roy, Serge Lebecque, et al.. Familial vs. sporadic sarcoidosis: BTNL2 polymorphisms, clinical presentations, and outcomes in a French cohort. Orphanet Journal of Rare Diseases, BioMed Central, 2016, 11 (1), 10.1186/s13023-016-0546-4. hal- 01595465 HAL Id: hal-01595465 https://hal.archives-ouvertes.fr/hal-01595465 Submitted on 26 Sep 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - ShareAlike| 4.0 International License Pacheco et al. Orphanet Journal of Rare Diseases (2016) 11:165 DOI 10.1186/s13023-016-0546-4 RESEARCH Open Access Familial vs. sporadic sarcoidosis: BTNL2 polymorphisms, clinical presentations, and outcomes in a French cohort Yves Pacheco1,11*, Alain Calender2, Dominique Israël-Biet3, Pascal Roy4, Serge Lebecque5, Vincent Cottin6, Diane Bouvry7, Hilario Nunes7, Pascal Sève8, Laurent Pérard9, Gilles Devouassoux8, Nathalie Freymond1, Chahira Khouatra6, Benoît Wallaert10, Raphaelle Lamy2, Mad-Hélénie Elsensohn4, Claire Bardel4, Dominique Valeyre7 and GSF group Abstract Background: The occurrence of familial forms of sarcoidosis (OMIM 181100) suggests a genetic predisposition. -

2027.Full.Pdf
Butyrophilin-like 2 Modulates B7 Costimulation To Induce Foxp3 Expression and Regulatory T Cell Development in Mature T Cells This information is current as of September 28, 2021. Ryan M. Swanson, Marc A. Gavin, Sabine S. Escobar, James B. Rottman, Brian P. Lipsky, Shishir Dube, Li Li, Jeannette Bigler, Martin Wolfson, Heather A. Arnett and Joanne L. Viney J Immunol 2013; 190:2027-2035; Prepublished online 28 Downloaded from January 2013; doi: 10.4049/jimmunol.1201760 http://www.jimmunol.org/content/190/5/2027 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2013/01/28/jimmunol.120176 Material 0.DC1 References This article cites 40 articles, 18 of which you can access for free at: http://www.jimmunol.org/content/190/5/2027.full#ref-list-1 by guest on September 28, 2021 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. -

Nº Ref Uniprot Proteína Péptidos Identificados Por MS/MS 1 P01024
Document downloaded from http://www.elsevier.es, day 26/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Nº Ref Uniprot Proteína Péptidos identificados 1 P01024 CO3_HUMAN Complement C3 OS=Homo sapiens GN=C3 PE=1 SV=2 por 162MS/MS 2 P02751 FINC_HUMAN Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 131 3 P01023 A2MG_HUMAN Alpha-2-macroglobulin OS=Homo sapiens GN=A2M PE=1 SV=3 128 4 P0C0L4 CO4A_HUMAN Complement C4-A OS=Homo sapiens GN=C4A PE=1 SV=1 95 5 P04275 VWF_HUMAN von Willebrand factor OS=Homo sapiens GN=VWF PE=1 SV=4 81 6 P02675 FIBB_HUMAN Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 78 7 P01031 CO5_HUMAN Complement C5 OS=Homo sapiens GN=C5 PE=1 SV=4 66 8 P02768 ALBU_HUMAN Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 66 9 P00450 CERU_HUMAN Ceruloplasmin OS=Homo sapiens GN=CP PE=1 SV=1 64 10 P02671 FIBA_HUMAN Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 58 11 P08603 CFAH_HUMAN Complement factor H OS=Homo sapiens GN=CFH PE=1 SV=4 56 12 P02787 TRFE_HUMAN Serotransferrin OS=Homo sapiens GN=TF PE=1 SV=3 54 13 P00747 PLMN_HUMAN Plasminogen OS=Homo sapiens GN=PLG PE=1 SV=2 48 14 P02679 FIBG_HUMAN Fibrinogen gamma chain OS=Homo sapiens GN=FGG PE=1 SV=3 47 15 P01871 IGHM_HUMAN Ig mu chain C region OS=Homo sapiens GN=IGHM PE=1 SV=3 41 16 P04003 C4BPA_HUMAN C4b-binding protein alpha chain OS=Homo sapiens GN=C4BPA PE=1 SV=2 37 17 Q9Y6R7 FCGBP_HUMAN IgGFc-binding protein OS=Homo sapiens GN=FCGBP PE=1 SV=3 30 18 O43866 CD5L_HUMAN CD5 antigen-like OS=Homo -

The Intracellular B30.2 Domain of Butyrophilin 3A1 Binds Phosphoantigens to Mediate Activation of Human Vg9vd2tcells
Immunity Article The Intracellular B30.2 Domain of Butyrophilin 3A1 Binds Phosphoantigens to Mediate Activation of Human Vg9Vd2TCells Andrew Sandstrom,1 Cassie-Marie Peigne´ ,2,3,4 Alexandra Le´ ger,2,3,4 James E. Crooks,5 Fabienne Konczak,2,3,4 Marie-Claude Gesnel,2,3,4 Richard Breathnach,2,3,4 Marc Bonneville,2,3,4 Emmanuel Scotet,2,3,4,* and Erin J. Adams1,6,* 1Department of Biochemistry and Molecular Biology, University of Chicago, Chicago, IL 60637, USA 2INSERM, Unite´ Mixte de Recherche 892, Centre de Recherche en Cance´ rologie Nantes Angers, 44000 Nantes, France 3University of Nantes, 44000 Nantes, France 4Centre National de la Recherche Scientifique (CNRS), Unite´ Mixte de Recherche 6299, 44000 Nantes, France 5Program in Biophysical Sciences, University of Chicago, Chicago, IL 60637, USA 6Committee on Immunology, University of Chicago, Chicago, IL 60637, USA *Correspondence: [email protected] (E.S.), [email protected] (E.J.A.) http://dx.doi.org/10.1016/j.immuni.2014.03.003 SUMMARY et al., 1999). In vitro, Vg9Vd2 T cells target certain cancer cell lines or cells treated with microbial extracts (Tanaka et al., 1994). In humans, Vg9Vd2 T cells detect tumor cells and mi- Vg9Vd2 T cell reactivity has been traced to accumulation of crobial infections, including Mycobacterium tubercu- organic pyrophosphate molecules commonly known as phos- losis, through recognition of small pyrophosphate phoantigens (pAgs) (Constant et al., 1994; Hintz et al., 2001; containing organic molecules known as phospho- Puan et al., 2007; Tanaka et al., 1995). These molecules are pro- antigens (pAgs). Key to pAg-mediated activation duced either endogenously, such as isopentenyl pyrophosphate of Vg9Vd2 T cells is the butyrophilin 3A1 (BTN3A1) (IPP), an intermediate of the mevalonate pathway in human cells that can accumulate intracellularly during tumorigenesis, or protein that contains an intracellular B30.2 domain by microbes, such as hydroxy-methyl-butyl-pyrophosphate critical to pAg reactivity. -

Functions to Inhibit T Cell Activation BTNL2, a Butyrophilin-Like
BTNL2, a Butyrophilin-Like Molecule That Functions to Inhibit T Cell Activation Thang Nguyen, Xikui K. Liu, Yongliang Zhang and Chen Dong This information is current as of October 2, 2021. J Immunol 2006; 176:7354-7360; ; doi: 10.4049/jimmunol.176.12.7354 http://www.jimmunol.org/content/176/12/7354 Downloaded from References This article cites 40 articles, 10 of which you can access for free at: http://www.jimmunol.org/content/176/12/7354.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on October 2, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2006 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology BTNL2, a Butyrophilin-Like Molecule That Functions to Inhibit T Cell Activation1 Thang Nguyen,2* Xikui K. Liu,2† Yongliang Zhang,† and Chen Dong3† B7 family members regulate T cell activation and tolerance. Although butyrophilin proteins share sequence homology with the B7 molecules, it is unclear whether they have any function in immune responses. -

2020 Zlatareva Iva 1521303 E
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ Tissue-specific butyrophilin-like proteins are TCR selecting ligands distinct from antigens Zlatareva, Iva Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 10. Oct. 2021 Tissue-specific butyrophilin-like proteins are gdTCR selecting ligands distinct from antigens Iva Ivanova Zlatareva King’s College London PhD Supervisors: Professor Adrian C.