FARE2017 WINNERS Sorted by Institute/Center
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Regulator of Aldosterone Synthesis in Human Adrenocortical Tissues
S J A FELIZOLA and others PCP4: a regulator of aldosterone 52:2 159–167 Research synthesis PCP4: a regulator of aldosterone synthesis in human adrenocortical tissues Saulo J A Felizola, Yasuhiro Nakamura, Yoshikiyo Ono1, Kanako Kitamura, Kumi Kikuchi1, Yoshiaki Onodera, Kazue Ise, Kei Takase2, Akira Sugawara3, Namita Hattangady4, William E Rainey4, Fumitoshi Satoh1 and Hironobu Sasano Department of Pathology, Tohoku University Graduate School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai, Correspondence Miyagi 980-8575, Japan should be addressed 1Division of Nephrology, Endocrinology and Vascular Medicine, Tohoku University Hospital, Sendai, Japan to Y Nakamura Departments of 2Diagnostic Radiology 3Molecular Endocrinology, Tohoku University Graduate School of Medicine, Email 2-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8575, Japan yasu-naka@ 4Department of Physiology and Medicine, University of Michigan, Ann Arbor, Michigan, USA patholo2.med.tohoku.ac.jp Abstract Purkinje cell protein 4 (PCP4) is a calmodulin (CaM)-binding protein that accelerates calcium Key Words association and dissociation with CaM. It has been previously detected in aldosterone- " Purkinje cell protein 4 (PCP4) producing adenomas (APA), but details on its expression and function in adrenocortical " adrenal cortex tissues have remained unknown. Therefore, we performed the immunohistochemical " aldosterone analysis of PCP4 in the following tissues: normal adrenal (NA; nZ15), APA (nZ15), cortisol- " calmodulin (CaM) producing adenomas (nZ15), and idiopathic hyperaldosteronism cases (IHA; nZ5). APA " CYP11B2 samples (nZ45) were also submitted to quantitative RT-PCR of PCP4, CYP11B1, and CYP11B2, Journal of Molecular Endocrinology as well as DNA sequencing for KCNJ5 mutations. Transient transfection analysis using PCP4 siRNA was also performed in H295R adrenocortical carcinoma cells, following ELISA analysis, and CYP11B2 luciferase assays were also performed after PCP4 vector transfection in order to study the regulation of PCP4 protein expression. -

A Comparative Analysis of Transcribed Genes in the Mouse Hypothalamus and Neocortex Reveals Chromosomal Clustering
A comparative analysis of transcribed genes in the mouse hypothalamus and neocortex reveals chromosomal clustering Wee-Ming Boon*, Tim Beissbarth†, Lavinia Hyde†, Gordon Smyth†, Jenny Gunnersen*, Derek A. Denton*‡, Hamish Scott†, and Seong-Seng Tan* *Howard Florey Institute, University of Melbourne, Parkville 3052, Australia; and †Genetics and Bioinfomatics Division, Walter and Eliza Hall Institute of Medical Research, Royal Parade, Parkville 3050, Australia Contributed by Derek A. Denton, August 26, 2004 The hypothalamus and neocortex are subdivisions of the mamma- representing all of the genes that are expressed (qualitative and lian forebrain, and yet, they have vastly different evolutionary quantitative) in the hypothalamus and neocortex under standard histories, cytoarchitecture, and biological functions. In an attempt conditions. to define these attributes in terms of their genetic activity, we have In the current study, we describe the use of the Serial Analysis compared their genetic repertoires by using the Serial Analysis of of Gene Expression (SAGE) database, which allows simulta- Gene Expression database. From a comparison of 78,784 hypothal- neous detection of the expression levels of the entire genome amus tags with 125,296 neocortical tags, we demonstrate that each without a priori knowledge of gene sequences (13). SAGE takes structure possesses a different transcriptional profile in terms of advantage of the fact that a small sequence tag taken from a gene ontological characteristics and expression levels. Despite its defined position within the transcript is sufficient to identify the more recent evolutionary history, the neocortex has a more com- gene (from known cDNA or EST sequences), and up to 40 tags plex pattern of gene activity. -

Mutations in Diphosphoinositol- Pentakisphosphate Kinase PPIP5K2 Are Associated with Hearing Loss in Human and Mouse
RESEARCH ARTICLE Mutations in Diphosphoinositol- Pentakisphosphate Kinase PPIP5K2 are associated with hearing loss in human and mouse Rizwan Yousaf1, Chunfang Gu2, Zubair M. Ahmed3, Shaheen N. Khan4, Thomas B. Friedman5, Sheikh Riazuddin6, Stephen B. Shears2, Saima Riazuddin1* a1111111111 1 Laboratory of Molecular Genetics, Department of Otorhinolaryngology-Head & Neck Surgery, School of Medicine University of Maryland, Baltimore, MD, United States of America, 2 Signal Transduction Laboratory, a1111111111 National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, a1111111111 NC, United States of America, 3 Laboratory of Neurogenetics and Regenerative Medicine, Department of a1111111111 Otorhinolaryngology-Head & Neck Surgery, School of Medicine University of Maryland, Baltimore, MD, United a1111111111 States of America, 4 National Center for Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan, 5 Section on Human Genetics, Laboratory of Molecular Genetics, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, MD, United States of America, 6 Shaheed Zulfiqar Ali Bhutto Medical University, Pakistan Institute of Medical Sciences, Islamabad, Pakistan * [email protected] OPEN ACCESS Citation: Yousaf R, Gu C, Ahmed ZM, Khan SN, Friedman TB, Riazuddin S, et al. (2018) Mutations Abstract in Diphosphoinositol-Pentakisphosphate Kinase PPIP5K2 are associated with hearing loss in Autosomal recessive nonsyndromic hearing loss is a genetically heterogeneous disorder. human and mouse. PLoS Genet 14(3): e1007297. https://doi.org/10.1371/journal.pgen.1007297 Here, we report a severe-to-profound sensorineural hearing loss locus, DFNB100 on chro- mosome 5q13.2-q23.2. Exome enrichment followed by massive parallel sequencing Editor: Karen B. Avraham, Tel Aviv University, ISRAEL revealed a c.2510G>A transition variant in PPIP5K2 that segregated with DFNB100-associ- ated hearing loss in two large apparently unrelated Pakistani families. -

Modulated in Intestinal Inflammation A
BTNL2, a Butyrophilin/B7-Like Molecule, Is a Negative Costimulatory Molecule Modulated in Intestinal Inflammation This information is current as Heather A. Arnett, Sabine S. Escobar, Eva Gonzalez-Suarez, of September 28, 2021. Alison L. Budelsky, Lori A. Steffen, Norman Boiani, Ming Zhang, Gerald Siu, Avery W. Brewer and Joanne L. Viney J Immunol 2007; 178:1523-1533; ; doi: 10.4049/jimmunol.178.3.1523 http://www.jimmunol.org/content/178/3/1523 Downloaded from References This article cites 40 articles, 12 of which you can access for free at: http://www.jimmunol.org/content/178/3/1523.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2007 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology BTNL2, a Butyrophilin/B7-Like Molecule, Is a Negative Costimulatory Molecule Modulated in Intestinal Inflammation Heather A. Arnett,1* Sabine S. -

Evidence for Differential Alternative Splicing in Blood of Young Boys With
Stamova et al. Molecular Autism 2013, 4:30 http://www.molecularautism.com/content/4/1/30 RESEARCH Open Access Evidence for differential alternative splicing in blood of young boys with autism spectrum disorders Boryana S Stamova1,2,5*, Yingfang Tian1,2,4, Christine W Nordahl1,3, Mark D Shen1,3, Sally Rogers1,3, David G Amaral1,3 and Frank R Sharp1,2 Abstract Background: Since RNA expression differences have been reported in autism spectrum disorder (ASD) for blood and brain, and differential alternative splicing (DAS) has been reported in ASD brains, we determined if there was DAS in blood mRNA of ASD subjects compared to typically developing (TD) controls, as well as in ASD subgroups related to cerebral volume. Methods: RNA from blood was processed on whole genome exon arrays for 2-4–year-old ASD and TD boys. An ANCOVA with age and batch as covariates was used to predict DAS for ALL ASD (n=30), ASD with normal total cerebral volumes (NTCV), and ASD with large total cerebral volumes (LTCV) compared to TD controls (n=20). Results: A total of 53 genes were predicted to have DAS for ALL ASD versus TD, 169 genes for ASD_NTCV versus TD, 1 gene for ASD_LTCV versus TD, and 27 genes for ASD_LTCV versus ASD_NTCV. These differences were significant at P <0.05 after false discovery rate corrections for multiple comparisons (FDR <5% false positives). A number of the genes predicted to have DAS in ASD are known to regulate DAS (SFPQ, SRPK1, SRSF11, SRSF2IP, FUS, LSM14A). In addition, a number of genes with predicted DAS are involved in pathways implicated in previous ASD studies, such as ROS monocyte/macrophage, Natural Killer Cell, mTOR, and NGF signaling. -
![Downloaded from [266]](https://docslib.b-cdn.net/cover/7352/downloaded-from-266-347352.webp)
Downloaded from [266]
Patterns of DNA methylation on the human X chromosome and use in analyzing X-chromosome inactivation by Allison Marie Cotton B.Sc., The University of Guelph, 2005 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in The Faculty of Graduate Studies (Medical Genetics) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) January 2012 © Allison Marie Cotton, 2012 Abstract The process of X-chromosome inactivation achieves dosage compensation between mammalian males and females. In females one X chromosome is transcriptionally silenced through a variety of epigenetic modifications including DNA methylation. Most X-linked genes are subject to X-chromosome inactivation and only expressed from the active X chromosome. On the inactive X chromosome, the CpG island promoters of genes subject to X-chromosome inactivation are methylated in their promoter regions, while genes which escape from X- chromosome inactivation have unmethylated CpG island promoters on both the active and inactive X chromosomes. The first objective of this thesis was to determine if the DNA methylation of CpG island promoters could be used to accurately predict X chromosome inactivation status. The second objective was to use DNA methylation to predict X-chromosome inactivation status in a variety of tissues. A comparison of blood, muscle, kidney and neural tissues revealed tissue-specific X-chromosome inactivation, in which 12% of genes escaped from X-chromosome inactivation in some, but not all, tissues. X-linked DNA methylation analysis of placental tissues predicted four times higher escape from X-chromosome inactivation than in any other tissue. Despite the hypomethylation of repetitive elements on both the X chromosome and the autosomes, no changes were detected in the frequency or intensity of placental Cot-1 holes. -

Noelia Díaz Blanco
Effects of environmental factors on the gonadal transcriptome of European sea bass (Dicentrarchus labrax), juvenile growth and sex ratios Noelia Díaz Blanco Ph.D. thesis 2014 Submitted in partial fulfillment of the requirements for the Ph.D. degree from the Universitat Pompeu Fabra (UPF). This work has been carried out at the Group of Biology of Reproduction (GBR), at the Department of Renewable Marine Resources of the Institute of Marine Sciences (ICM-CSIC). Thesis supervisor: Dr. Francesc Piferrer Professor d’Investigació Institut de Ciències del Mar (ICM-CSIC) i ii A mis padres A Xavi iii iv Acknowledgements This thesis has been made possible by the support of many people who in one way or another, many times unknowingly, gave me the strength to overcome this "long and winding road". First of all, I would like to thank my supervisor, Dr. Francesc Piferrer, for his patience, guidance and wise advice throughout all this Ph.D. experience. But above all, for the trust he placed on me almost seven years ago when he offered me the opportunity to be part of his team. Thanks also for teaching me how to question always everything, for sharing with me your enthusiasm for science and for giving me the opportunity of learning from you by participating in many projects, collaborations and scientific meetings. I am also thankful to my colleagues (former and present Group of Biology of Reproduction members) for your support and encouragement throughout this journey. To the “exGBRs”, thanks for helping me with my first steps into this world. Working as an undergrad with you Dr. -

Prioritization of Metabolic Genes As Novel Therapeutic Targets in Estrogen-Receptor Negative Breast Tumors Using Multi-Omics Data and Text Mining
www.oncotarget.com Oncotarget, 2019, Vol. 10, (No. 39), pp: 3894-3909 Research Paper Prioritization of metabolic genes as novel therapeutic targets in estrogen-receptor negative breast tumors using multi-omics data and text mining Dinesh Kumar Barupal1,*, Bei Gao1,*, Jan Budczies2, Brett S. Phinney4, Bertrand Perroud4, Carsten Denkert2,3 and Oliver Fiehn1 1West Coast Metabolomics Center, University of California, Davis, CA, USA 2Institute of Pathology, Charité University Hospital, Berlin, Germany 3German Institute of Pathology, Philipps-University Marburg, Marburg, Germany 4UC Davis Genome Center, University of California, Davis, CA, USA *Co-first authors and contributed equally to this work Correspondence to: Oliver Fiehn, email: [email protected] Keywords: set-enrichment; ChemRICH; multi-omics; metabolic networks; candidate gene prioritization Received: March 12, 2019 Accepted: May 13, 2019 Published: June 11, 2019 Copyright: Barupal et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Estrogen-receptor negative (ERneg) breast cancer is an aggressive breast cancer subtype in the need for new therapeutic options. We have analyzed metabolomics, proteomics and transcriptomics data for a cohort of 276 breast tumors (MetaCancer study) and nine public transcriptomics datasets using univariate statistics, meta- analysis, Reactome pathway analysis, biochemical network mapping and text mining of metabolic genes. In the MetaCancer cohort, a total of 29% metabolites, 21% proteins and 33% transcripts were significantly different (raw p <0.05) between ERneg and ERpos breast tumors. -
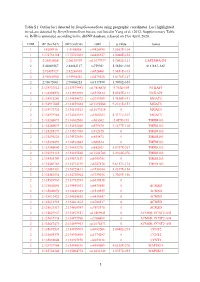
1 Table S1. Outlier Loci Detected by Deepgenomescan Using
Table S1. Outlier loci detected by DeepGenomeScan using geographic coordinates. Loci highlighted in red are detected by DeepGenomeScan but are not listed in Yang et al. (2012; Supplementary Table 4). RsID is annotated according to the dbSNP database released on 21st April, 2020. CHR BP (Grch37) BP (Grch38) rsID p.value Genes 1 1:4208918 1:4148858 rs9426495 3.8582E-104 1 1:175738168 1:175769032 rs6425357 2.0088E-105 2 2:20310668 2:20110907 rs11679737 1.2002E-111 LAPTM4A-DT 2 2:40289267 2:40062127 rs759361 5.5658E-104 SLC8A1-AS1 2 2:82495127 2:82268003 rs6726401 3.9451E-103 2 2:96660300 2:95994552 rs2579520 1.8176E-127 2 2:96672001 2:96006253 rs1917890 3.7698E-104 2 2:134333012 2:133575441 rs17816830 9.702E-105 NCKAP5 2 2:134350570 2:133592999 rs6715224 5.8745E-111 NCKAP5 2 2:134912243 2:134154672 rs2139309 1.7418E-191 MGAT5 2 2:134917005 2:134159434 rs11692586 9.2121E-151 MGAT5 2 2:134972732 2:134215161 rs11679218 0 MGAT5 2 2:134979966 2:134222395 rs1965183 5.3171E-107 MGAT5 2 2:135260071 2:134502500 rs503562 2.057E-153 TMEM163 2 2:135280039 2:134522468 rs579670 3.1477E-118 TMEM163 2 2:135285279 2:134527708 rs512375 0 TMEM163 2 2:135290221 2:134532650 rs655472 0 TMEM163 2 2:135290453 2:134532882 rs666614 0 TMEM163 2 2:135340840 2:134583270 rs842361 5.6717E-237 TMEM163 2 2:135393110 2:134635540 rs11684785 2.2164E-276 TMEM163 2 2:135430709 2:134673139 rs6745983 0 TMEM163 2 2:135469769 2:134712199 rs6747870 9.6117E-114 TMEM163 2 2:135483381 2:134725811 rs3739034 5.0357E-154 2 2:135483534 2:134725964 rs3739036 3.7269E-156 2 2:135539967 2:134782397 -

Identification of Differentially Expressed Genes in Human Bladder Cancer Through Genome-Wide Gene Expression Profiling
521-531 24/7/06 18:28 Page 521 ONCOLOGY REPORTS 16: 521-531, 2006 521 Identification of differentially expressed genes in human bladder cancer through genome-wide gene expression profiling KAZUMORI KAWAKAMI1,3, HIDEKI ENOKIDA1, TOKUSHI TACHIWADA1, TAKENARI GOTANDA1, KENGO TSUNEYOSHI1, HIROYUKI KUBO1, KENRYU NISHIYAMA1, MASAKI TAKIGUCHI2, MASAYUKI NAKAGAWA1 and NAOHIKO SEKI3 1Department of Urology, Graduate School of Medical and Dental Sciences, Kagoshima University, 8-35-1 Sakuragaoka, Kagoshima 890-8520; Departments of 2Biochemistry and Genetics, and 3Functional Genomics, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan Received February 15, 2006; Accepted April 27, 2006 Abstract. Large-scale gene expression profiling is an effective CKS2 gene not only as a potential biomarker for diagnosing, strategy for understanding the progression of bladder cancer but also for staging human BC. This is the first report (BC). The aim of this study was to identify genes that are demonstrating that CKS2 expression is strongly correlated expressed differently in the course of BC progression and to with the progression of human BC. establish new biomarkers for BC. Specimens from 21 patients with pathologically confirmed superficial (n=10) or Introduction invasive (n=11) BC and 4 normal bladder samples were studied; samples from 14 of the 21 BC samples were subjected Bladder cancer (BC) is among the 5 most common to microarray analysis. The validity of the microarray results malignancies worldwide, and the 2nd most common tumor of was verified by real-time RT-PCR. Of the 136 up-regulated the genitourinary tract and the 2nd most common cause of genes we detected, 21 were present in all 14 BCs examined death in patients with cancer of the urinary tract (1-7). -

Identifying Genetic Risk Factors for Coronary Artery Angiographic Stenosis in a Genetically Diverse Population
Please do not remove this page Identifying Genetic Risk Factors for Coronary Artery Angiographic Stenosis in a Genetically Diverse Population Liu, Zhi https://scholarship.miami.edu/discovery/delivery/01UOML_INST:ResearchRepository/12355224170002976?l#13355497430002976 Liu, Z. (2016). Identifying Genetic Risk Factors for Coronary Artery Angiographic Stenosis in a Genetically Diverse Population [University of Miami]. https://scholarship.miami.edu/discovery/fulldisplay/alma991031447280502976/01UOML_INST:ResearchR epository Embargo Downloaded On 2021/09/26 20:05:11 -0400 Please do not remove this page UNIVERSITY OF MIAMI IDENTIFYING GENETIC RISK FACTORS FOR CORONARY ARTERY ANGIOGRAPHIC STENOSIS IN A GENETICALLY DIVERSE POPULATION By Zhi Liu A DISSERTATION Submitted to the Faculty of the University of Miami in partial fulfillment of the requirements for the degree of Doctor of Philosophy Coral Gables, Florida August 2016 ©2016 Zhi Liu All Rights Reserved UNIVERSITY OF MIAMI A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy IDENTIFYING GENETIC RISK FACTORS FOR CORONARY ARTERY ANGIOGRAPHIC STENOSIS IN A GENETICALLY DIVERSE POPULATION Zhi Liu Approved: ________________ _________________ Gary W. Beecham, Ph.D. Liyong Wang, Ph.D. Assistant Professor of Human Associate Professor of Human Genetics Genetics ________________ _________________ Eden R. Martin, Ph.D. Guillermo Prado, Ph.D. Professor of Human Genetics Dean of the Graduate School ________________ Tatjana Rundek, M.D., Ph.D. Professor of Neurology LIU, ZHI (Ph.D., Human Genetics and Genomics) Identifying Genetic Risk Factors for Coronary Artery (August 2016) Angiographic Stenosis in a Genetically Diverse Population Abstract of a dissertation at the University of Miami. Dissertation supervised by Professor Gary W. -

Next Generation Exome Sequencing of Paediatric Inflammatory Bowel Disease Patients Identifies Rare and Novel Variants in Candida
Gut Online First, published on April 28, 2012 as 10.1136/gutjnl-2011-301833 Inflammatory bowel disease ORIGINAL ARTICLE Gut: first published as 10.1136/gutjnl-2011-301833 on 28 April 2012. Downloaded from Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes Katja Christodoulou,1 Anthony E Wiskin,2 Jane Gibson,1 William Tapper,1 Claire Willis,2 Nadeem A Afzal,3 Rosanna Upstill-Goddard,1 John W Holloway,4 Michael A Simpson,5 R Mark Beattie,3 Andrew Collins,1 Sarah Ennis1 < Additional materials are ABSTRACT published online only. To view Background Multiple genes have been implicated by Significance of this study these files please visit the association studies in altering inflammatory bowel journal online (http://gut.bmj. com/content/early/recent). disease (IBD) predisposition. Paediatric patients often What is already known on this subject? manifest more extensive disease and a particularly < For numbered affiliations see Genome-wide association studies have impli- end of article. severe disease course. It is likely that genetic cated numerous candidate genes for inflamma- predisposition plays a more substantial role in this group. tory bowel disease (IBD), but evidence of Correspondence to Objective To identify the spectrum of rare and novel causality for specific variants is largely absent. Dr Sarah Ennis, Genetic variation in known IBD susceptibility genes using exome Furthermore, by design, genome-wide associa- Epidemiology and Genomic sequencing analysis in eight individual cases of childhood Informatics Group, Human tion studies are limited to the study of Genetics, Faculty of Medicine, onset severe disease.