Pathogenesis of Hemolysis Anemia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Hemolysis and Venous Thrombosis: Which Link? A
ISSN: 2378-3656 Rkiouak et al. Clin Med Rev Case Rep 2020, 7:329 DOI: 10.23937/2378-3656/1410329 Volume 7 | Issue 11 Clinical Medical Reviews Open Access and Case Reports ORIGINAL RESEARCH Hemolysis and Venous Thrombosis: Which Link? A. Rkiouak, PH.D1, I El Kassimi, MD2, N Sahel, MD2, M Zaizaa, MD2 and Y Sekkach, PhD1 Check for updates Internal Medicine A Department, Mohammed V Military Hospital Medical School of Rabat, Morocco *Corresponding author: Adil Rkiouak, PH.D., Department of Internal Medicine A, Mohammed V Military Hospital, Medical School of Rabat, Morocco, Tel: +212-66-179-44-04 The mechanism of antibody-mediated hemolysis is Abstract via phagocytosis or complement-mediated destruction The association hemolysis and venous thrombosis remains and can occur intravascular or extravascular. The intra- unknown to clinicians, despite our advances in comrehen- sion of pathophysiological bases. vascular mechanisms include direct cellular destruction via lysis, toxins, or trauma; fragmentation and oxida- Haemolysis, which is observed in multiple diseases, can affect all three components of Virchow’s triad. It is not sur- tion. prising that there is a link between haemolytic disorders and Multiple haemolytic disorders produce substantial thrombosis. intravascular haemolysis. Examples, the corpuscular We will try to clarify the main pro-thrombotic mechanisms hemolysis include PNH, extra-corpuscular haemolysis, during hemolysis through 3 clinical observations of deep ve- acquired (autoimmune haemolytic anaemia (AIHA , nous thrombosis in 3 main types of hemolytic pathologies, ) namely a case of paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura (PTT)), as well thrombotic thrombocytopenic purpura and autoimmune as other diseases. These disorders are also associated anemia hemolytic. -

Clostridial Septicemia with Intravascular Hemolysis: a Case Report G
Henry Ford Hospital Medical Journal Volume 13 | Number 4 Article 4 12-1965 Clostridial Septicemia With Intravascular Hemolysis: A Case Report G. M. Mastio E. Morfin Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Mastio, G. M. and Morfin, E. (1965) "Clostridial Septicemia With Intravascular Hemolysis: A Case Report," Henry Ford Hospital Medical Bulletin : Vol. 13 : No. 4 , 421-425. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol13/iss4/4 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Bull. Vol. 13, December 1965 CLOSTRIDIAL SEPTICEMIA WITH INTRAVASCULAR HEMOLYSIS A CASE REPORT G. M. MASTIC, M.D. AND E. MORFIN, M.D. In 1871 Bottini' demonstrated the bacterial nature of gas gangrene, but failed to isolate a causal organism. Clostridium perfringens, sometimes known as Clostridium welchii, was discovered independently during 1892 and 1893 by Welch, Frankel, "Veillon and Zuber.^ This organism is a saprophytic inhabitant of the intestinal tract, and may be a harmless saprophyte of the female genital tract occurring in the vagina in 4-6 per cent of pregnant women. Clostridial organisms occur in great numbers and distribution throughout the world. Because of this, they are very common in traumatic wounds. Very few species of Clostridia, however, are pathogenic, and still fewer are capable of producing gas gangrene in man. -

Transfusion Problems in Hemolytic Anemias*
Transfusion Problems in Hemolytic Anemias* ALI A. HOSSAIN! Department of Pathdlogy, Medical College of Virginia, Richmond 23219 All hemolytic anemias feature shortened red cell Extrinsic Mechanisms survival due to premature hemolysis of the cell. For the Those hemolytic anemias which are due to extrinsic purposes of this presentation, we may classify the factors may be classified, further, as non-immune or hemolytic anemias, most broadly, according to the immune. Non-immune mechanisms include a) drugs mechanisms leading to hemolysis. and chemicals (phenylhydrazine, naphthalene, lead, snake venoms); b) physical agents (heat); c) bacteria Intrinsic Mechanisms and parasites (hemolytic streptococci, Clostridium Hemolytic anemias due to intrinsically defective welchii, Bartonella, plasmodia); and d) acquired sen erythrocytes are essentially of three types. First are sitivity to penicillin, methylodopa, Keftin®, or fava those anemias in which the red cells are defective due plant as examples. Some of the agents in this. last to lack of an essential factor, eg, pernicious anemia group serve to lyse the cells, either through duect in relapse. The second type includes those in which action or by formation of antibodies. the red cells have an abnormal shape because of an These hemolytic anemias due to extrinsic factors inherited error in the chemical makeup of the hemo of the non-immune variety present no transfusion globin molecules; eg, sickle cells, elliptocytes, sphero problem for the Blood Bank. However, it ~~st b.e cytes, and the target -
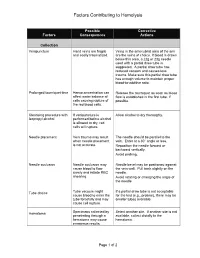
Factors Contributing to Hemolysis
Factors Contributing to Hemolysis Possible Corrective Factors Consequences Actions Collection Venipuncture Hand veins are fragile Veins in the antecubital area of the arm and easily traumatized. are the veins of choice. If blood is drawn below this area, a 22g or 23g needle used with a partial draw tube is suggested. A partial draw tube has reduced vacuum and causes less trauma. Make sure this partial draw tube has enough volume to maintain proper blood-to-additive ratio. Prolonged tourniquet time Hemoconcentration can Release the tourniquet as soon as blood affect water balance of flow is established in the first tube, if cells causing rupture of possible. the red blood cells. Cleansing procedure with If venipuncture is Allow alcohol to dry thoroughly. isopropyl alcohol performed before alcohol is allowed to dry, red cells will rupture. Needle placement Vein trauma may result The needle should be parallel to the when needle placement vein. Enter at a 30° angle or less. is not accurate. Reposition the needle forward or backward vertically. Avoid probing. Needle occlusion Needle occlusion may Needle bevel may be positioned against cause blood to flow the vein wall. Pull back slightly on the slowly and initiate RBC needle. shearing. Avoid rotating or changing the angle of the needle. Tube choice Tube vacuum might If a partial draw tube is not acceptable cause blood to enter the for the test (e.g., protime), there may be tube forcefully and may smaller tubes available. cause cell rupture. Hematoma Specimens collected by Select another site. If another site is not penetrating through a available, collect distally to the hematoma may cause hematoma. -

Hemolytic Disease of the Newborn
Intensive Care Nursery House Staff Manual Hemolytic Disease of the Newborn INTRODUCTION and DEFINITION: Hemolytic Disease of the Newborn (HDN), also known as erythroblastosis fetalis, isoimmunization, or blood group incompatibility, occurs when fetal red blood cells (RBCs), which possess an antigen that the mother lacks, cross the placenta into the maternal circulation, where they stimulate antibody production. The antibodies return to the fetal circulation and result in RBC destruction. DIFFERENTIAL DIAGNOSIS of hemolytic anemia in a newborn infant: -Isoimmunization -RBC enzyme disorders (e.g., G6PD, pyruvate kinase deficiency) -Hemoglobin synthesis disorders (e.g., alpha-thalassemias) -RBC membrane abnormalities (e.g., hereditary spherocytosis, elliptocytosis) -Hemangiomas (Kasabach Merritt syndrome) -Acquired conditions, such as sepsis, infections with TORCH or Parvovirus B19 (anemia due to RBC aplasia) and hemolysis secondary to drugs. ISOIMMUNIZATION A. Rh disease (Rh = Rhesus factor) (1) Genetics: Rh positive (+) denotes presence of D antigen. The number of antigenic sites on RBCs varies with genotype. Prevalence of genotype varies with the population. Rh negative (d/d) individuals comprise 15% of Caucasians, 5.5% of African Americans, and <1% of Asians. A sensitized Rh negative mother produces anti-Rh IgG antibodies that cross the placenta. Risk factors for antibody production include 2nd (or later) pregnancies*, maternal toxemia, paternal zygosity (D/D rather than D/d), feto-maternal compatibility in ABO system and antigen load. (2) Clinical presentation of HDN varies from mild jaundice and anemia to hydrops fetalis (with ascites, pleural and pericardial effusions). Because the placenta clears bilirubin, the chief risk to the fetus is anemia. Extramedullary hematopoiesis (due to anemia) results in hepatosplenomegaly. -

A Newly Recognized Blood Group in Domestic Shorthair Cats: the Mik Red Cell Antigen
J Vet Intern Med 2007;21:287–292 A Newly Recognized Blood Group in Domestic Shorthair Cats: The Mik Red Cell Antigen Nicole M. Weinstein, Marie-Claude Blais, Kimberly Harris, Donna A. Oakley, Lillian R. Aronson, and Urs Giger Background: Naturally occurring alloantibodies produced against A and B red cell antigens in cats can cause acute hemolytic transfusion reactions. Blood incompatibilities, unrelated to the AB blood group system, have also been suspected after blood transfusions through routine crossmatch testing or as a result of hemolytic transfusion reactions. Hypothesis: Incompatible crossmatch results among AB compatible cats signify the presence of a naturally occurring alloantibody against a newly identified blood antigen in a group of previously never transfused blood donor cats. The associated alloantibody is clinically important based upon a hemolytic transfusion reaction after inadvertent transfusion of red cells expressing this red cell antigen in a feline renal transplant recipient that lacks this red cell antigen. Methods: Blood donor and nonblood donor cats were evaluated for the presence of auto- and alloantibodies using direct antiglobulin and crossmatch tests, respectively, and were blood typed for AB blood group status. Both standard tube and novel gel column techniques were used. Results: Plasma from 3 of 65 cats and 1 feline renal transplant recipient caused incompatible crossmatch test results with AB compatible erythrocytes indicating these cats formed an alloantibody against a red cell antigen they lack, termed Mik. The 3 donors and the renal transplant recipient were crossmatch-compatible with one another. Tube and gel column crossmatch test results were similar. Conclusions and Clinical Importance: The absence of this novel Mik red cell antigen can be associated with naturally occurring anti-Mik alloantibodies and can elicit an acute hemolytic transfusion reaction after an AB-matched blood transfusion. -

A Suspected Case of Clostridium Perfringens Sepsis With
Uojima et al. Journal of Medical Case Reports (2019) 13:125 https://doi.org/10.1186/s13256-019-2023-x CASE REPORT Open Access A suspected case of Clostridium perfringens sepsis with intravascular hemolysis after transhepatic arterial chemoembolization: a case report Haruki Uojima1,2*, Mie Onoue2, Hisashi Hidaka2, Naohisa Wada2, Yoshiaki Tanaka2, Tomoyoshi Inoue2, Kousuke Kubota2, Takahide Nakazawa2, Akitaka Shibuya2 and Wasaburo Koizumi2 Abstract Introduction: Sepsis due to Clostridium perfringens, one of several clostridial species, is an important cause of massive intravascular hemolysis in patients with underlying malignancies. Chronic liver diseases, immunosuppression, and presence of malignancies were risk factors for Clostridium perfringens sepsis. Therefore, Clostridium perfringens sepsis should always be considered in patients presenting with liver damage after chemo- embolic therapy for hepatocellular carcinoma. This case report focuses on findings characteristic of an intravascular hemolysis due to Clostridium perfringens after transhepatic arterial chemoembolization. Case presentation: An 83-year-old Japanese man presented to our hospital because of a third recurrence of hepatocellular carcinoma. He had nonalcoholic steatohepatitis-related cirrhosis, and underwent radiofrequency ablation and transhepatic arterial chemoembolization therapy for hepatocellular carcinoma of S4/S8 and S2. He had a medical history of pancreatic carcinoma and underwent pylorus-preserving pancreaticoduodenectomy approximately 5 years ago. Because follow-up -

Blood Bank I D
The Osler Institute Blood Bank I D. Joe Chaffin, MD Bonfils Blood Center, Denver, CO The Fun Just Never Ends… A. Blood Bank I • Blood Groups B. Blood Bank II • Blood Donation and Autologous Blood • Pretransfusion Testing C. Blood Bank III • Component Therapy D. Blood Bank IV • Transfusion Complications * Noninfectious (Transfusion Reactions) * Infectious (Transfusion-transmitted Diseases) E. Blood Bank V (not discussed today but available at www.bbguy.org) • Hematopoietic Progenitor Cell Transplantation F. Blood Bank Practical • Management of specific clinical situations • Calculations, Antibody ID and no-pressure sample questions Blood Bank I Blood Groups I. Basic Antigen-Antibody Testing A. Basic Red Cell-Antibody Interactions 1. Agglutination a. Clumping of red cells due to antibody coating b. Main reaction we look for in Blood Banking c. Two stages: 1) Coating of cells (“sensitization”) a) Affected by antibody specificity, electrostatic RBC charge, temperature, amounts of antigen and antibody b) Low Ionic Strength Saline (LISS) decreases repulsive charges between RBCs; tends to enhance cold antibodies and autoantibodies c) Polyethylene glycol (PEG) excludes H2O, tends to enhance warm antibodies and autoantibodies. 2) Formation of bridges a) Lattice structure formed by antibodies and RBCs b) IgG isn’t good at this; one antibody arm must attach to one cell and other arm to the other cell. c) IgM is better because of its pentameric structure. P}Chaffin (12/28/11) Blood Bank I page 1 Pathology Review Course 2. Hemolysis a. Direct lysis of a red cell due to antibody coating b. Uncommon, but equal to agglutination. 1) Requires complement fixation 2) IgM antibodies do this better than IgG. -

Autoimmune Hemolytic Anemia in COVID-19 Patients, the « Transmissible » Direct Coombs Test
J H C R JOURNAL OF HEMATOLOGY 2640-2823 AND CLINICAL RESEARCH Research Article More Information *Address for Correspondence: Alice Brochier, Hematology Department of Laboratory Medicine, Autoimmune hemolytic anemia in Saint-Luc University Hospital, Avenue Hippocrate 10, 1200 Brussels, Belgium, Tel: +322764 6814; COVID-19 patients, the « transmissible » Email: [email protected]; Véronique Deneys, Hematology Department of Laboratory Medicine, Saint-Luc University direct Coombs test Hospital, Avenue Hippocrate 10, 1200 Brussels, Belgium, Email: [email protected] Alice Brochier1*, Julien Cabo1, Claudine Guerrieri1, Leïla Belkhir2, Submitted: March 24, 2021 3 1 Pierre-François Laterre and Véronique Deneys * Approved: April 06, 2021 Published: April 07, 2021 1Hematology Department of Laboratory Medicine, Saint-Luc University Hospital, Brussels, Belgium 2Department of Internal Medicine and Infectious Diseases, Saint-Luc University Hospital, Brussels, How to cite this article: Brochier A, Cabo J, Guerrieri C, Belkhir L, Laterre PF, Deneys V. Belgium Autoimmune hemolytic anemia in COVID-19 3 Department of Intensive Care Medicine, Saint-Luc University Hospital, Brussels, Belgium patients, the « transmissible » direct Coombs test. J Hematol Clin Res. 2021; 5: 004-008. Abstract DOI: 10.29328/journal.jhcr.1001016 Copyright: © 2021 Brochier A, et al. This Background: Like other viruses, the SARS-CoV-2 (severe acute respiratory syndrome is an open access article distributed under coronavirus 2) appears to be responsible for several autoimmune complications. The occurrence the Creative Commons Attribution License, of autoimmune hemolytic anemia has been described in several case reports. This AIHA was also which permits unrestricted use, distribution, noticeable by the important number of blood transfusions required for COVID-19 (coronavirus and reproduction in any medium, provided the disease 2019) patients. -

Delayed Hemolytic Transfusion Reaction/Hyperhemolysis Syndrome in Children with Sickle Cell Disease
Delayed Hemolytic Transfusion Reaction/Hyperhemolysis Syndrome in Children With Sickle Cell Disease Julie-An M. Talano, MD*ʈ; Cheryl A. Hillery, MD*§ʈ; Jerome L. Gottschall, MD‡§ʈ; Diane M. Baylerian, BS, MT§ʈ; and J. Paul Scott, MD*§ʈ ABSTRACT. Objective. Alloimmunization in patients lower than it was at the time of original transfusion, with sickle cell disease (SCD) has a reported incidence of suggesting the hemolysis of the patient’s own RBCs in 5% to 36%. One complication of alloimmunization is addition to hemolysis of the transfused RBCs; a negative delayed hemolytic transfusion reaction/hyperhemolysis DAT and reticulocytopenia are often present. Severe (DHTR/H) syndrome, which has a reported incidence of complications including acute chest syndrome, conges- 11%. In patients with SCD, clinical findings in DHTR/H tive heart failure, pancreatitis, and acute renal failure syndrome occur approximately 1 week after the red were associated with DHTR/H syndrome in our patients. blood cell (RBC) transfusion and include the onset of DHTR/H in the pediatric sickle cell population is a seri- increased hemolysis associated with pain and profound ous and potentially life-threatening complication of RBC anemia. The hemoglobin (Hb) often drops below pre- transfusion. It is important to avoid additional trans- transfusion levels. In many reported adult cases, the di- fusions in these patients, if possible, because these may rect antiglobulin test (DAT) remains negative and no exacerbate the hemolysis and worsen the degree of new alloantibody is detected as the cause for these trans- anemia. DHTR/H syndrome must be included in the fusion reactions. -

Immune Hemolytic Anemia in a Patient with Plasmodium Vivax Malaria
Journal Home Page www.bbbulletin.org BRITISH BIOMEDICAL BULLETIN Original Immune Hemolytic Anemia in a Patient with Plasmodium vivax Malaria Deepak Nayak M.*, Sushma V. Belurkar and Anna Joseph Amprayil Department of Pathology, Kasturba Medical College-Manipal, Manipal University. Manipal 576104, India A R T I C L E I N F O A B S T R A C T Received 14 May. 2014 Received in revised form 06 June. 2014 Accepted 10 June. 2014 The combination of anemia in malarial infestations has been well documented in literature. But an immune hemolytic anemia developing within days of treatment for Plasmodium vivax malaria Keywords : Plasmodium vivax; has seldom been reported. We present a case of a patient with Hemolytic anemia; vivax malaria who developed severe anemia and jaundice on day Jaundice. seven of initiating treatment with artesunate; necessitating expedient measures. This case highlights the importance, yet under-reported association of Plasmodium vivax malaria and immune-mediated hemolysis. Corresponding author:Deepak Nayak M. Department of Pathology, Kasturba Medical College-Manipal, Manipal University. Manipal 576104, India . E-mail address: © 2014 British Biomedical Bulletin. All rights reserved [email protected] Nayak M. et al__________________________________________________ ISSN-2347-5447 Introduction Coomb’s test was advised to ascertain an Malaria continues to be an important immune-mediated hemolysis; which showed disease in India. The varied presentations of a weak positivity for direct antibodies. the disease and its diversity in terms of Clinically, the patient was afebrile but pale. hematological manifestations have been well The biochemical tests showed a raised endowed in literature. By and large, anemia unconjugated bilirubin (6.3mg/dl) which seen in malaria is multifactorial. -

Hemolysis Transforms Liver Macrophages Into Anti-Inflammatory Erythrophagocytes
Hemolysis transforms liver macrophages into anti-inflammatory erythrophagocytes Marc Pfefferlé, … , Dominik J. Schaer, Florence Vallelian J Clin Invest. 2020. https://doi.org/10.1172/JCI137282. Research In-Press Preview Hematology Graphical abstract Find the latest version: https://jci.me/137282/pdf Pfefferle et al. Hemolysis induced anti-inflammatory erythrophagocytes Hemolysis transforms liver macrophages into anti-inflammatory erythrophagocytes 1 1 2 1 1 Marc Pfefferlé , Giada Ingoglia , Christian A. Schaer , Ayla Yalamanoglu , Raphael Buzzi , 1 3 3 1 1 Irina L. Dubach , Ge Tan , Emilio Y. López-Cano , Nadja Schulthess , Kerstin Hansen , Rok 1 1 1 Humar , Dominik J. Schaer and Florence Vallelian 1 Division of Internal Medicine, University of Zurich, Switzerland 2 Institute of Anesthesiology, University of Zurich, Switzerland 3 Functional Genomics Center Zurich, ETH Zurich/University of Zurich, Switzerland MP and GI contributed equally to this work. DJS and FV share last authorship. Keywords: phagocytes, erythrophagocytosis, heme, hemolysis, inflammation, innate immunity, non-alcoholic fatty liver disease Corresponding Authors: Dominik J. Schaer, MD Division of Internal Medicine University Hospital CH-8091 Zurich Switzerland +41 44 255 2382 [email protected] Florence Vallelian, MD Division of Internal Medicine University Hospital CH-8091 Zurich Switzerland +41 44 255 1697 [email protected] 1 Pfefferle et al. Hemolysis induced anti-inflammatory erythrophagocytes GRAPHICAL ABSTRACT