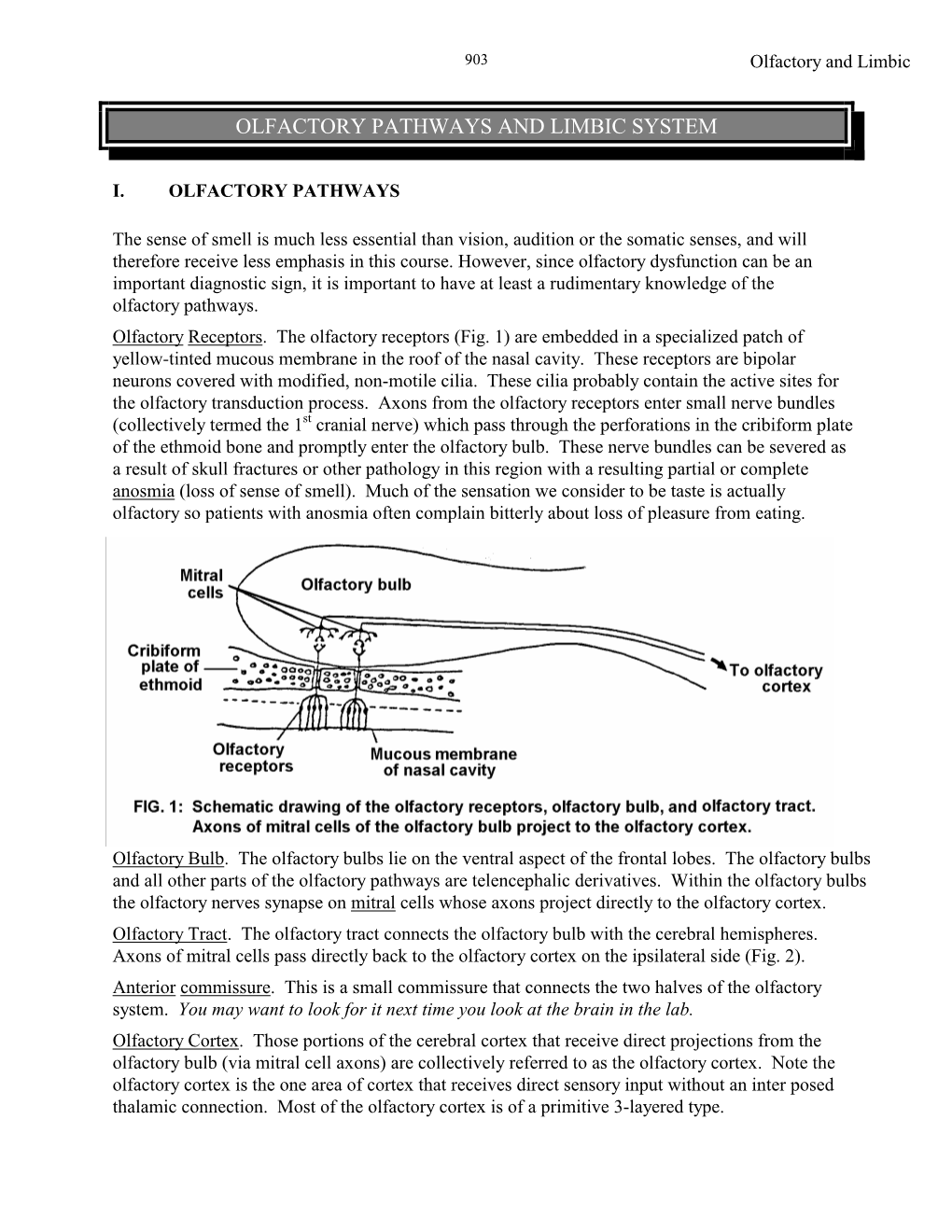
Olfactory Pathways and Limbic System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Born Granule Cells During Implicit Versus Explicit Olfactory Learning
RESEARCH ARTICLE Opposite regulation of inhibition by adult- born granule cells during implicit versus explicit olfactory learning Nathalie Mandairon1*, Nicola Kuczewski1, Florence Kermen1, Je´ re´ my Forest1, Maellie Midroit1, Marion Richard1, Marc Thevenet1, Joelle Sacquet1, Christiane Linster2,3, Anne Didier1 1Lyon Neuroscience Research Center, Neuroplasticity and Neuropathology of Olfactory Perception Team, CNRS UMR 5292, INSERM U1028, Universite´ de Lyon, Lyon, France; 2Computational Physiology Lab, Cornell University, Ithaca, United States; 3Department of Neurobiology and Behavior, Cornell University, Ithaca, United States Abstract Both passive exposure and active learning through reinforcement enhance fine sensory discrimination abilities. In the olfactory system, this enhancement is thought to occur partially through the integration of adult-born inhibitory interneurons resulting in a refinement of the representation of overlapping odorants. Here, we identify in mice a novel and unexpected dissociation between passive and active learning at the level of adult-born granule cells. Specifically, while both passive and active learning processes augment neurogenesis, adult-born cells differ in their morphology, functional coupling and thus their impact on olfactory bulb output. Morphological analysis, optogenetic stimulation of adult-born neurons and mitral cell recordings revealed that passive learning induces increased inhibitory action by adult-born neurons, probably resulting in more sparse and thus less overlapping odor representations. Conversely, after active learning inhibitory action is found to be diminished due to reduced connectivity. In this case, strengthened odor response might underlie enhanced discriminability. *For correspondence: DOI: https://doi.org/10.7554/eLife.34976.001 [email protected] Competing interests: The authors declare that no Introduction competing interests exist. Brain representations of the environment constantly evolve through learning mediated by different Funding: See page 13 plasticity mechanisms. -

Microstructural White Matter Alterations and Hippocampal Volumes Are
6 178 S Fjalldal and others Diffusion tensor imaging in 178:6 577–587 Clinical Study craniopharyngioma Microstructural white matter alterations and hippocampal volumes are associated with cognitive deficits in craniopharyngioma S Fjalldal1, C Follin1, D Svärd2, L Rylander3, S Gabery4, Å Petersén4, D van Westen2, P C Sundgren2,5, I M Björkman-Burtscher2,5, J Lätt5, B Ekman6, A Johanson7 and E M Erfurth1 1Department of Endocrinology, Skåne University Hospital, Lund, Sweden, 2Department of Diagnostic Radiology, Clinical Sciences, 3Division of Occupational and Environmental Medicine, 4Translational Neuroendocrine Research Unit, Department 5 of Experimental Medical Science, Lund University, Lund, Sweden, Department of Medical Imaging and Physiology, Correspondence 6 Skåne University Hospital, Lund, Sweden, Department of Endocrinology and Medical and Health Sciences, should be addressed 7 Linköping University, Linköping, Sweden, and Department of Psychology and Psychiatry, Skåne University Hospital, to E M Erfurth Lund, Sweden Email [email protected] Abstract Context: Patients with craniopharyngioma (CP) and hypothalamic lesions (HL) have cognitive deficits. Which neural pathways are affected is unknown. Objective: To determine whether there is a relationship between microstructural white matter (WM) alterations detected with diffusion tensor imaging (DTI) and cognition in adults with childhood-onset CP. Design: A cross-sectional study with a median follow-up time of 22 (6–49) years after operation. Setting: The South Medical Region of Sweden (2.5 million inhabitants). Participants: Included were 41 patients (24 women, ≥17 years) surgically treated for childhood-onset CP between 1958–2010 and 32 controls with similar age and gender distributions. HL was found in 23 patients. Main outcome measures: Subjects performed cognitive tests and magnetic resonance imaging, and images were analyzed using DTI of uncinate fasciculus, fornix, cingulum, hippocampus and hypothalamus as well as hippocampal European Journal European of Endocrinology volumetry. -

From Human Emotions to Robot Emotions
1 American Association for Artificial Intelligence – Spring Symposium 3/2004, Stanford University – Keynote Lecture. From Human Emotions to Robot Emotions Jean-Marc Fellous The Salk Institute for Neurobiological Studies 10010 N. Torrey Pines Road, la Jolla, CA 92037 [email protected] Abstract1 open a new window on the neural bases of emotions that may offer new ways of thinking about implementing robot- The main difficulties that researchers face in understanding emotions. emotions are difficulties only because of the narrow- mindedness of our views on emotions. We are not able to Why are emotions so difficult to study? free ourselves from the notion that emotions are necessarily human emotions. I will argue that if animals have A difficulty in studying human emotions is that here are emotions, then so can robots. Studies in neuroscience have significant individual differences, based on experiential as shown that animal models, though having limitations, have well as genetic factors (Rolls, 1998; Ortony, 2002; significantly contributed to our understanding of the Davidson, 2003a, b; Ortony et al., 2004). My fear at the functional and mechanistic aspects of emotions. I will sight of a bear may be very different from the fear suggest that one of the main functions of emotions is to experienced by a park-ranger who has a better sense for achieve the multi-level communication of simplified but high impact information. The way this function is achieved bear-danger and knows how to react. My fear might also be in the brain depends on the species, and on the specific different from that of another individual who has had about emotion considered. -

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -

Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment
The Journal of Neuroscience, December 1989, 9(12): 4355-4370 Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment Stuart Zola-Morgan,’ Larry Ft. Squire,’ David G. Amaral,2 and Wendy A. Suzuki2J Veterans Administration Medical Center, San Diego, California, 92161, and Department of Psychiatry, University of California, San Diego, La Jolla, California 92093, The Salk Institute, San Diego, California 92136, and 3Group in Neurosciences, University of California, San Diego, La Jolla, California 92093 In monkeys, bilateral damage to the medial temporal region Moss, 1984). (In this notation, H refers to the hippocampus, A produces severe memory impairment. This lesion, which in- to the amygdala, and the plus superscript (+) to the cortical cludes the hippocampal formation, amygdala, and adjacent tissue adjacent to each structure.) This lesion appears to con- cortex, including the parahippocampal gyrus (the H+A+ le- stitute an animal model of medial temporal lobe amnesia like sion), appears to constitute an animal model of human me- that exhibited by the well-studied patient H.M. (Scoville and dial temporal lobe amnesia. Reexamination of histological Milner, 1957). material from previously studied monkeys with H+A+ lesions The H+A+ lesion produces greater memory impairment than indicated that the perirhinal cortex had also sustained sig- a lesion limited to the hippocampal formation and parahip- nificant damage. Furthermore, recent neuroanatomical stud- pocampal cortex-the H+ lesion (Mishkin, 1978; Mahut et al., ies show that the perirhinal cortex and the closely associated 1982; Zola-Morgan and Squire, 1985, 1986; Zola-Morgan et al., parahippocampal cortex provide the major source of cortical 1989a). -

Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: a Steered-Glutamatergic Perspective
brain sciences Review Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: A Steered-Glutamatergic Perspective Amjad H. Bazzari * and H. Rheinallt Parri School of Life and Health Sciences, Aston University, Birmingham B4 7ET, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-(0)1212044186 Received: 7 October 2019; Accepted: 29 October 2019; Published: 31 October 2019 Abstract: The molecular pathways underlying the induction and maintenance of long-term synaptic plasticity have been extensively investigated revealing various mechanisms by which neurons control their synaptic strength. The dynamic nature of neuronal connections combined with plasticity-mediated long-lasting structural and functional alterations provide valuable insights into neuronal encoding processes as molecular substrates of not only learning and memory but potentially other sensory, motor and behavioural functions that reflect previous experience. However, one key element receiving little attention in the study of synaptic plasticity is the role of neuromodulators, which are known to orchestrate neuronal activity on brain-wide, network and synaptic scales. We aim to review current evidence on the mechanisms by which certain modulators, namely dopamine, acetylcholine, noradrenaline and serotonin, control synaptic plasticity induction through corresponding metabotropic receptors in a pathway-specific manner. Lastly, we propose that neuromodulators control plasticity outcomes through steering glutamatergic transmission, thereby gating its induction and maintenance. Keywords: neuromodulators; synaptic plasticity; learning; memory; LTP; LTD; GPCR; astrocytes 1. Introduction A huge emphasis has been put into discovering the molecular pathways that govern synaptic plasticity induction since it was first discovered [1], which markedly improved our understanding of the functional aspects of plasticity while introducing a surprisingly tremendous complexity due to numerous mechanisms involved despite sharing common “glutamatergic” mediators [2]. -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

What Can Be Learned from White Matter Alterations in Antisocial Girls Willeke M
Menks WM, Raschle NM. J Neurol Neuromedicine (2017) 2(7): 16-20 Neuromedicine www.jneurology.com www.jneurology.com Journal of Neurology & Neuromedicine Mini Review Open Access What can be learned from white matter alterations in antisocial girls Willeke M. Menks1, Christina Stadler1 and Nora M. Raschle1 1Department of Child and Adolescent Psychiatry, University of Basel, Psychiatric University Hospital Basel, Switzerland. Article Info ABSTRACT Article Notes Antisocial behavior in youths constitutes a major public health problem Received: June 17, 2017 worldwide. Conduct disorder is a severe variant of antisocial behavior with higher Accepted: July 31, 2017 prevalence rates for boys (12%) as opposed to girls (7%). A better understanding *Correspondence: of the underlying neurobiological mechanisms of conduct disorder is warranted Dr. Willeke Menks, PhD to improve identification, diagnosis, or treatment. Functional and structural Department of Child and Adolescent Psychiatry (KJPK), neuroimaging studies have indicated several key brain regions within the limbic Psychiatric University Clinics Basel (UPK) system and prefrontal cortex that are altered in youths with conduct disorder. Schanzenstrasse 13, CH-4056 Basel, Switzerland Examining the structural connectivity, i.e. white matter fiber tracts connecting Tel. +41 61 265 89 76 these brain areas, may further inform about the underlying neural mechanisms. Fax +41 61 265 89 61 Diffusion tensor imaging (DTI) is a non-invasive technique that can evaluate the © 2017 Menks WM & Raschle NM. This article is distributed white matter integrity of fiber tracts throughout the brain. To date, DTI studies have under the terms of the Creative Commons Attribution 4.0 found several white matter tracts that are altered in youths with conduct disorder. -

Distinct Representations of Olfactory Information in Different Cortical Centres
LETTER doi:10.1038/nature09868 Distinct representations of olfactory information in different cortical centres Dara L. Sosulski1, Maria Lissitsyna Bloom1{, Tyler Cutforth1{, Richard Axel1 & Sandeep Robert Datta1{ Sensory information is transmitted to the brain where it must be behaviours, but is unlikely to specify innate behaviours. Rather, innate processed to translate stimulus features into appropriate beha- olfactory behaviours are likely to result from the activation of genetically vioural output. In the olfactory system, distributed neural activity determined, stereotyped neural circuits. We have therefore developed a in the nose is converted into a segregated map in the olfactory strategy to trace the projections from identified glomeruli in the olfactory bulb1–3. Here we investigate how this ordered representation is bulb to higher olfactory cortical centres. transformed in higher olfactory centres in mice. We have Mitral and tufted cells that innervate a single glomerulus were developed a tracing strategy to define the neural circuits that labelled by electroporation of tetramethylrhodamine (TMR)-dextran convey information from individual glomeruli in the olfactory under the guidance of a two-photon microscope. This technique labels bulb to the piriform cortex and the cortical amygdala. The spatial mitral and tufted cells that innervate a single glomerulus and is suffi- order in the bulb is discarded in the piriform cortex; axons from ciently robust to allow the identification of axon termini within mul- individual glomeruli project diffusely to the piriform without tiple higher order olfactory centres (Figs 1a–c, 2 and Supplementary apparent spatial preference. In the cortical amygdala, we observe Figs 1–4). Labelling of glomeruli in the olfactory bulbs of mice that broad patches of projections that are spatially stereotyped for indi- express GFP under the control of specific odorant receptor promoters vidual glomeruli. -

Cranial Nerve Palsy
Cranial Nerve Palsy What is a cranial nerve? Cranial nerves are nerves that lead directly from the brain to parts of our head, face, and trunk. There are 12 pairs of cranial nerves and some are involved in special senses (sight, smell, hearing, taste, feeling) while others control muscles and glands. Which cranial nerves pertain to the eyes? The second cranial nerve is called the optic nerve. It sends visual information from the eye to the brain. The third cranial nerve is called the oculomotor nerve. It is involved with eye movement, eyelid movement, and the function of the pupil and lens inside the eye. The fourth cranial nerve is called the trochlear nerve and the sixth cranial nerve is called the abducens nerve. They each innervate an eye muscle involved in eye movement. The fifth cranial nerve is called the trigeminal nerve. It provides facial touch sensation (including sensation on the eye). What is a cranial nerve palsy? A palsy is a lack of function of a nerve. A cranial nerve palsy may cause a complete or partial weakness or paralysis of the areas served by the affected nerve. In the case of a cranial nerve that has multiple functions (such as the oculomotor nerve), it is possible for a palsy to affect all of the various functions or only some of the functions of that nerve. What are some causes of a cranial nerve palsy? A cranial nerve palsy can occur due to a variety of causes. It can be congenital (present at birth), traumatic, or due to blood vessel disease (hypertension, diabetes, strokes, aneurysms, etc). -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925).