Good's Buffers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tropix Catalog.Indd 1 3/23/07 12:43:35 PM 1 Chemiluminescent Substrates and Chemiluminescent Enhancers
2007 Chemiluminescent Product Guide Tropix catalog.indd 1 3/23/07 12:43:35 PM 1 Chemiluminescent Substrates and Chemiluminescent Enhancers 1 Chemiluminescent Substrates and Chemiluminescent Enhancers . .1 2 Reporter Gene Assays and Reagents . .9 3 Immunodetection Products . 25 4 Nucleic Acid Membrane-Based Detection Products . 38 5 Reagents and Accessories for Chemiluminescence. 43 Introduction . .1 CDP-Star® Substrate and CSPD® Substrates . .3 Galacton® / Galacton-Plus® / Galacton-Star® Substrates . .4 Glucuron® Substrate . .5 Glucon™ Substrate . .5 NA-Star® Substrate. .6 Solution-based Luminescence Enhancers Sapphire™, Sapphire-II™, Emerald™, Emerald-II™, and Ruby™ . .7 Membrane-based Luminescence Enhancers Nitro-Block™ and Nitro-Block-II™. .8 ii Tropix catalog.indd 2 3/23/07 12:43:39 PM Substrates and Enhancers Introduction OO OCH 3 Chemiluminescence ® CDP-Star Chemiluminescence is the conversion of chemical energy to light energy. Cl Cl = OPO Several different chemical reactions, including some enzyme-catalyzed reac- Alkaline 3 tions, result in the production of visible light. Chemiluminescence reactions Phosphatase occur naturally (bioluminescence) in a wide variety of organisms, including OO OCH 3 beetles, jellyfish, bacteria, and many marine organisms. In addition, there Metastable Intermediate are several classes of synthetic chemical structures that upon chemical or Cl Cl enzymatic cleavage produce light emission. Chemiluminescent reactions O - are employed in a wide variety of applications, including but not limited to OCH 3 O biological assays, clinical diagnostic assays, biosensors, hygiene monitoring, O O - * and commercial low-level lighting. Cl Cl Principles of Enzyme-activated Chemiluminescence 1,2-Dioxetane substrates emit visible light upon enzyme-catalyzed decom- Light position. Chemiluminescent detection of biomolecules with 1,2-dioxetane Figure 1. -

The Role of 3-Deoxy-D-Arabino-Heptulosonate 7- Phosphate Synthase 1 in Arabidopsis Thaliana Metabolism
THE ROLE OF 3-DEOXY-D-ARABINO-HEPTULOSONATE 7- PHOSPHATE SYNTHASE 1 IN ARABIDOPSIS THALIANA METABOLISM by Jimmy Poulin A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Cell and System Biology University of Toronto © Copyright by Jimmy Poulin, 2011 The role of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase 1 in Arabidopsis thaliana metabolism Jimmy Poulin Master of Science Cell and System Biology University of Toronto 2011 Abstract The enzyme 3-deoxy-D-arabino-heptulusonate 7-phosphate synthase (DHS) catalyzes the first step of the shikimate pathway. In bacteria, the regulation of the pathway is mediated by allosteric inhibition of DHS by the aromatic amino acids tyrosine, phenylalanine and tryptophan. The regulation of the pathway in plants remains elusive but the aromatic amino acids are involved as suggested by the hypersensitivity of dhs1 knockout mutant to tyrosine. In this study the effects of the dhs1 mutation on endogenous levels of aromatic amino acids and of downstream metabolites are explored. HPLC analysis is used to measure levels of tyrosine and phenylalanine and 5-methyltryptophan sensitivity is used to probe levels of tryptophan. Additionally, the auxin content of whole seedlings was quantified by LC/MS and its local levels at the root apex are visualized with the DR5::GUS reporter system. ii Acknowledgements I could not have completed my master’s degree without the help of many resourceful individuals. First and foremost I would like to thank Dr. Dinesh Christendat for his supervision and guidance. I am also grateful for guidance received from Dr. -

2014 SRS Program
SRS 2014 STUDENT April 11-12 RESEARCH University of Hawai‘i at Ma¯noa SYMPOSIUM Proudly presented by the University of Hawai‘i at Ma¯noa College Tropical Agriculture and Human Resources and College of Engineering Welcome to the University of Hawai‘i at Mānoa’s College of Tropical Agriculture and Human Resources (CTAHR) and College of Engineering (COE) 2014 Student Research Symposium. This annual event, now in its 26th year, brings together graduate and undergraduate students to share the research they are pursuing under the supervision of faculty in CTAHR and COE. The students are able to present their findings, exchange information, and incorporate what they have learned from their peers into their own scholarly work. The scientific exploration and engineering design conducted by students in CTAHR and COE is truly multidisciplinary, and the Student Research Symposium reflects this diversity and the strong relationship between CTAHR and COE. The investigations presented here range from fundamental studies to novel applications and encompass engineering, production agriculture, environmental technologies, health and food sciences, family and consumer sciences, and natural sciences. All stages of the research and development process and multiple types of student learning experiences are represented: discovery; advanced diagnostics and laboratory testing; design, validation, and field testing; and adoption of new methods and technologies. Each project represents a unique path that contributes to CTAHR’s mission of preparing students for life in the global community through research that fosters viable communities, a diversified economy, and a healthy environment, as well as COE’s mission of providing research experiences and opportunities to students that will enhance the growth of the technological workforce and stimulate the growth of technology-based industries in Hawai‘i. -

1. What Are the Benefits and Limitation Gene
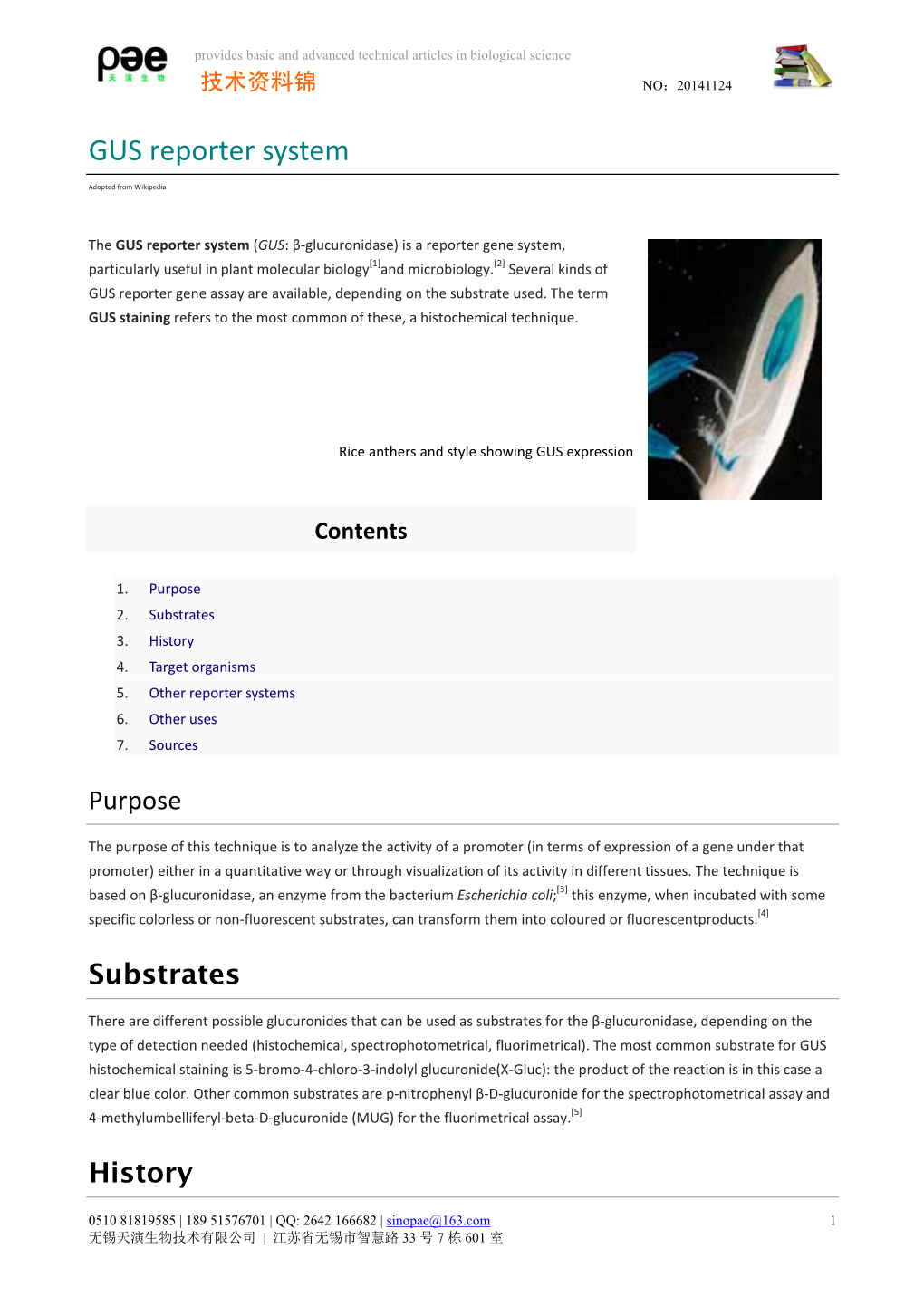
COMPILED AND CIRCULATED BY BANGAMOTI HANSDA, ASSISTANT PROFESSOR, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE GUS 1. What are the benefits and limitations of the GUS Gene Reporter System in Plants? Gene reporters enable valuable insight into gene expression. The GUS gene reporter system is one of the popular and common plant reporter systems. GUS is short for glucuronidase, an enzyme in the bacterium E. coli. GUS is a good reporter for plants, as it does not occur naturally, and thus, has a low background. With some simple genetic techniques, one can attach the promoter of the gene you want to investigate to the GUS coding region. You can then transform your reporter construct into your plant species of choice to monitor its expression. Transformation can be accomplished in plants via methods like Agrobaterium-mediated gene transfer. The GUS assay does not require the presence of any cofactors or ions for function. Beta- glucuronidase can function through a wide range of pH values, and is fairly resistant to thermal inactivation. However, GUS is susceptible to inhibition from certain heavy metal ions, such as Cu2+ and Zn2+. Additionally, the interpretation of the assay is limited by the movement of diX-indigo throughout the cell. DiX-indigo, can associate with lipids to diffuse far from the site of enzyme activity, which shows a lack of cytosolic localization and irregularity of substrate penetration. This can potentially lead to an incorrect interpretation of GUS protein localization. Despite a lack of cellular localization, nuclear localization of GUS has been well observed. GUS assays can be carried out in the presence of potassium ferricyanide to prevent the stain from diffusing. -

An Efficient Protocol for Root Studies in the Common Sunflower Using Composite Plants Tyler Parks Eastern Illinois University
Eastern Illinois University The Keep Masters Theses Student Theses & Publications 2018 An Efficient Protocol for Root Studies in the Common Sunflower Using Composite Plants Tyler Parks Eastern Illinois University Recommended Citation Parks, Tyler, "An Efficient Protocol for Root Studies in the Common Sunflower Using Composite Plants" (2018). Masters Theses. 4405. https://thekeep.eiu.edu/theses/4405 This Thesis is brought to you for free and open access by the Student Theses & Publications at The Keep. It has been accepted for inclusion in Masters Theses by an authorized administrator of The Keep. For more information, please contact [email protected]. TheGraduate School� EAITTJ\Nlwl'OS lJNJVERS1rr Thesis Maintenance and Reproduction Certificate FOR: Graduate Candidates Completing Theses in Partial Fulfillment of the Degree Graduate Faculty Advisors Directing the Theses RE: Preservation, Reproduction, and Distribution ofThesis Research Preserving, reproducing, and distributing thesis research Is an important part of Booth Library's responsibility to provide access to scholarship. In order to further this goal, Booth Library makes all graduate theses completed as part of a degree program at Eastern Illinois University available for personal study, research, and other not-for profit educational purposes. Under 17 U.S.C. § 108, the library may reproduce and distribute a copy without infringing on copyright; however, professional courtesy dictates that permission be requested from the author before doing so. Your signatures affirm the following: •The graduate candidate is the author of this thesis. •The graduate candidate retains the copyright and intellectual property rights associated with the original research, creative activity, and intellectual or artistic content of the thesis. -

Assessment Three Constitutive Promoters for GUS Expression in Rice (Oryza Sativa L., Var. J-104)
ARTÍCULO DE INVESTIGACIÓN Assessment three constitutive promoters for GUS expression in rice (Oryza sativa L., var. J-104) Evaluación de tres promotores constitutivos para la expresión GUS en arroz (Oryza sativa L., cv. J-104) Maylin Pérez Bernal*, Daymí Abreu Remedios**, Onel Valdivia Pérez***, Magalis Delgado Rigo****, Raúl Armas Ramos***** DOI: 10.15446/rev.colomb.biote.v18n1.57716 Abstract This work analyzed the constitutive expression of the ß-Glucuronidase (GUS) reporter gene fused to three promoters: the cauliflower mosaic virus (CaMV) 35S, the chimerical A9 promoter which contains rice Act1, and the Ubiquitine-1 promoter from maize. The activity of the promoters was qualitative and quantitatively obtained in different tissues and various growth stages of rice plants (cv J-104) transformed by biolistic. All the promoters were found to be active, with distinct patterns of relative activity in leaves, stem and roots from in vitro and ex vitro plants, and in plants of T1 progeny. The chimerical A9 promoter increased significantly levels of GUS expression in all the tissues and at all growth stages of the plants. Key words: CaMV 35S, chimerical A9 promoter, ubiquitine-1. Resumen Se analizó la expresión constitutiva del gen reportero de la ß-Glucuronidasa (GUS) fusionado a tres promotores: el 35S del virus del mosaico de la coliflor (CaMV), el promotor quimérico A9 que contiene la actina-1 de arroz y el promotor ubiquitina-1 de maíz. La actividad de los promotores fue analizada cualitativa y cuantitativamente en diferentes tejidos y estadíos de crecimiento de plantas de arroz (variedad J-104) transformadas mediante biobalística. Se demostró la expresión constitutiva de GUS bajo los promotores estudiados, con distintos patrones de actividad relativa en hojas, tallos y raíces de plantas in vitro y ex vitro, y en plantas de la progenie T1. -

An Examination of Leaf Morphogenesis in the Moss, Physcomitrella Patens, in an Oral Examination Held on August 30, 2011
AN EXAMINATION OF LEAF MORPHOGENESIS IN THE MOSS, PHYSCOMITRELLA PATENS A Thesis Submitted to the Faculty of Graduate Studies and Research In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy In Biology University of Regina By Elizabeth Io Barker Regina, Saskatchewan August, 2011 Copyright 2011: E.I. Barker UNIVERSITY OF REGINA FACULTY OF GRADUATE STUDIES AND RESEARCH SUPERVISORY AND EXAMINING COMMITTEE Elizabeth Barker, candidate for the degree of Doctor of Philosophy in Biology, has presented a thesis titled, An Examination of Leaf Morphogenesis In The Moss, Physcomitrella Patens, in an oral examination held on August 30, 2011. The following committee members have found the thesis acceptable in form and content, and that the candidate demonstrated satisfactory knowledge of the subject material. External Examiner: Dr. David Cove, University of Leeds Supervisor: Dr. Neil Ashton, Department of Biology Committee Member: Dr. Harold Weger, Department of Biology Committee Member: Dr. William Chapco, Department of Biology Committee Member: *Dr. Janis Dale, Department of Geology Chair of Defense: Dr. Philip Charrier. Department of History *Not present at defense ABSTRACT Physcomitrella patens is a simple model plant belonging to the bryophytes, which diverged from the tracheophytes approximately 500 million years ago. The leaves of the moss are similar in form to vascular plant leaves although leaves evolved independently in the bryophyte and tracheophyte lineages. Close examination of the morphology of Physcomitrella leaves and investigation of the morphogenetic processes that result in the leaf form and of the hormonal and genetic regulation of those processes will elucidate the evolutionary trajectory of moss leaves. -

Richard Jefferson
Richard Jefferson Science as Social Enterprise The CAMBIA BiOS Initiative Nearly four billion people live on daily incomes lower than the price of a latté at Starbucks. Most of them make dramatically less than that—and from that income, they must acquire their food, their medicine, their shelter and clothing, their edu- cation, and their recreation, and they must build their future and their dreams. Their lives, and the quality of their lives, hinge on biological innovation. Biological innovation is the ability to harness living systems for our social, environmental and economic well-being. It is the oldest and most fundamental form of human innovation, involving as it does the getting of food, the striving for health, the making of homes, and the building of communities. The wealth creat- ed over the millennia through the domestication and husbandry of plants and ani- mals has powered human society. Of all areas of biological innovation, agriculture is the most important, affect- ing our environment, our health, our economies, and the fabric of our societies. The world’s poorest nations depend largely on agriculture for their economic sur- vival as well as their food, fuel and fiber. The challenges of innovation to create and sustain productive and environmentally sound agriculture are even more pro- nounced in these societies. Any failure to do so has enormous implications for the global community, over and above the social, economic, and environmental impacts. For thousands of years biological innovation has been informed and guided by keen observation and the accumulation and sharing of generations of empirical knowledge. -

Moss (Physcomitrella Patens) GH3 Proteins Act in Auxin Homeostasis
Research MossBlackwellOxford,NPHNew0028-646X1469-8137©267710.1111/j.1469-8137.2008.02677.xOctober0323???338???OriginalXX The Phytologist Authors UK 2008Article Publishing (2008). Ltd Journal compilation © New Phytologist( (2008)Physcomitrella patens) XX GH3 proteins act in auxin homeostasis Jutta Ludwig-Müller1, Sabine Jülke1, Nicole M. Bierfreund2, Eva L. Decker2 and Ralf Reski2,3 1Institute of Botany, Technische Universität Dresden, D–01062 Dresden, Germany; 2Plant Biotechnology, Faculty of Biology, University of Freiburg, Schänzlestrasse 1, D–79104 Freiburg, Germany; 3Centre for Biological Signalling Studies (bioss), University of Freiburg, Schänzlestrasse 1, D–79104 Freiburg, Germany Summary Author for correspondence: • Auxins are hormones involved in many cellular, physiological and developmental J. Ludwig-Müller processes in seed plants and in mosses such as Physcomitrella patens. Control of + Tel: 49 351 463 33939 auxin levels is achieved in higher plants via synthesis of auxin conjugates by Fax: +49 351 463 37032 Email: Jutta.Ludwig-Mueller@ members of the GH3 family. The role of the two GH3-like proteins from P. patens tu-dresden.de for growth and auxin homeostasis was therefore analysed. •The in vivo-function of the two P. patens GH3 genes was investigated using Received: 31 July 2008 Accepted: 19 September 2008 single and double knockout mutants. The two P. patens GH3 proteins were also heterologously expressed to determine their enzymatic activity. • Both P. patens GH3 enzymes accepted the auxin indole acetic acid (IAA) as New Phytologist (2009) 181: 323–338 doi: 10.1111/j.1469-8137.2008.02677.x substrate, but with different preferences for the amino acid to which it is attached. Cytoplasmic localization was shown for PpGH3-1 tagged with green fluorescent protein (GFP). -

Thirty Years of Plant Transformation
Thirty years of plant transformation A case study exploring the impact of plant transformation technology on plant science research and the global agricultural biotechnology industry Credits, left to right, Dominik Maenni, Jason Yardley, Toony, US Agricultural Research Service Thirty years of plant transformation In 1983 researchers demonstrated that they could insert role in identifying the genetic basis of important crop new genes into a plant genome, using a species of soil characteristics, which breeders are incorporating into Impact Summary bacteria called Agrobacterium tumefaciens. new varieties with increased yield, disease resistance AFRC-funded research at the Plant Breeding or which produce healthier, more nutritious food. Institute (PBI), Cambridge, published in 1983 The breakthrough was made simultaneously by three Much of this work relies on genetic tools developed by enabled researchers to create transgenic research groups, including a team at the Plant Breeding researchers at PBI. plants using Agrobacterium-mediated plant Institute (PBI) in Cambridge UK, which was funded transformation. by BBSRC’s predecessor the Agricultural and Food Plant transformation technology also led to the creation Research Council (AFRC). The genetic tools developed of the agricultural biotechnology industry, which in at PBI became freely available to academics, ensuring 2012 had a global market value of US$14.84Bn1. The the technique was adopted by research groups around genetically modified crops produced by this industry are The technology, including novel vectors and the world. now grown in countries such as the USA, Brazil, India reporter genes developed at PBI, revolutionised and China, although different regulations and on-going research around the world, and now forms a Since then, the technology, known as ‘Agrobacterium- public debate mean few are grown in Europe. -

Analysis of Cyclodipeptide Biosynthetic Genes in Nocardiopsis Alba ATCC BAA-2165
Analysis of Cyclodipeptide Biosynthetic Genes in Nocardiopsis alba ATCC BAA-2165 A thesis presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Master of Science Yongli Li May 2014 © 2014 Yongli Li. All Rights Reserved. 2 This thesis titled Analysis of Cyclodipeptide Biosynthetic Genes in Nocardiopsis alba ATCC BAA-2165 by YONGLI LI has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Shawn Chen Assistant Professor of Biological Sciences Robert Frank Dean, College of Arts and Sciences 3 ABSTRACT LI, YONGLI., M.S., May 2014, Biological Sciences Analysis of Cyclodipeptide Biosynthetic Genes in Nocardiopsis alba ATCC BAA-2165 Director of Thesis: Shawn Chen Nocardiopsis alba ATCC BAA-2165 is an actinobacterium isolated from honeybee guts in Southern Ohio. It was reported N. alba showed antibiotic activity against several Gram-positive microorganisms, including two honeybee pathogens. Bioactivity-guided compound isolation led to an identification of two cyclodipeptides, albonoursin (cyclo(ΔPhe-ΔLeu)) and its analog (cyclo(mΔTyr-ΔLeu)), as the bioactive metabolites produced by N. alba. Despite its important environmental presence, characterization of the Nocardiopsis genus was limited due to the lack of genetic tools. In this project, we focused on the cyclodipeptides production of N. alba to establish a system for genetic analysis of Nocardiopsis. An albonoursin cyclodipeptide biosynthetic gene cluster, albABC, was identified in the N. alba genome. A PCR-targeting strategy was developed to generate an albABC deletion mutant of N. alba; the mutant, YL001, was shown to have lost the production of cyclodipeptides. -

Evaluation of GM Trees Expressing a Fungal Enzyme Kandidatarbeten I
Kandidatarbeten 2019:3 i skogsvetenskap Fakulteten för skogsvetenskap Evaluation of GM trees expressing a fungal enzyme - Investigating the effects of the fungal enzyme on activation of jasmonate signaling using JAZ10:GUS reporter lines of Arabidopsis Utvärdering av GM-träd som uttrycker svampenzym - Undersöker effekten av svampenzymer effekt på aktiveringen av jasmonatsignalering med hjälp av JAZ10:GUS reporterlinje i Arabidopsis Elin Bäckström & Therese Thalin Sveriges Lantbruksuniversitet Självständigt arbete i skogsvetenskap, 15 hp Jägmästarprogrammet Umeå Kandidatarbeten i Skogsvetenskap Fakulteten för skogsvetenskap, Sveriges lantbruksuniversitet Institutionen för skogens ekologi och skötsel Enhet/Unit Department of Forest Ecology and Management Författare/Author Elin Bäckström & Therese Thalin Utvärdering av GM-träd som uttrycker svampenzym - Undersöker effekten av svampenzymer effekt på Titel, Sv aktiveringen av jasmonatsignalering med hjälp av JAZ10:GUS reporterlinje i Arabidopsis Evaluation of GM trees expressing a fungal enzyme - Investigating the effects of the fungal enzyme on Titel, Eng activation of jasmonate signaling using JAZ10:GUS reporter lines of Arabidopsis Cellväggsbildning, cellväggskomposition, Nyckelord/ genmodifiering, genetisk förädling, GUS/ cell wall Keywords formation, cell wall composition, genetic modification, genetic improvement, GUS Ewa Mellerowicz, UPSC, Dept Forest Genetics and Handledare/Supervisor Plant Physiology, /Institutionen för skoglig genetik och växtfysiologi Tommy Mörling Examinator/Examiner