Foundations of Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
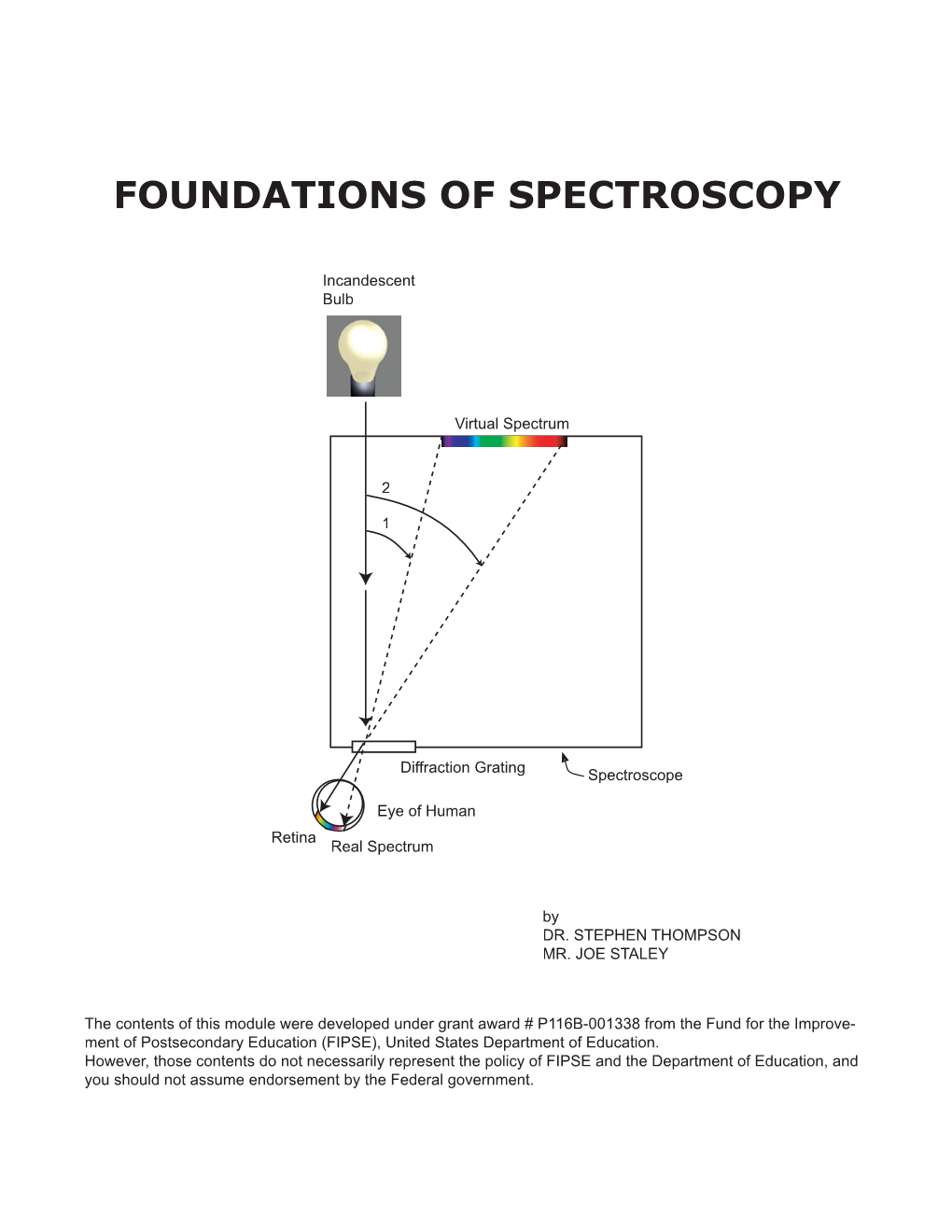
Load more
Recommended publications
-

The Electromagnetic Spectrum
The Electromagnetic Spectrum Wavelength/frequency/energy MAP TAP 2003-2004 The Electromagnetic Spectrum 1 Teacher Page • Content: Physical Science—The Electromagnetic Spectrum • Grade Level: High School • Creator: Dorothy Walk • Curriculum Objectives: SC 1; Intro Phys/Chem IV.A (waves) MAP TAP 2003-2004 The Electromagnetic Spectrum 2 MAP TAP 2003-2004 The Electromagnetic Spectrum 3 What is it? • The electromagnetic spectrum is the complete spectrum or continuum of light including radio waves, infrared, visible light, ultraviolet light, X- rays and gamma rays • An electromagnetic wave consists of electric and magnetic fields which vibrates thus making waves. MAP TAP 2003-2004 The Electromagnetic Spectrum 4 Waves • Properties of waves include speed, frequency and wavelength • Speed (s), frequency (f) and wavelength (l) are related in the formula l x f = s • All light travels at a speed of 3 s 108 m/s in a vacuum MAP TAP 2003-2004 The Electromagnetic Spectrum 5 Wavelength, Frequency and Energy • Since all light travels at the same speed, wavelength and frequency have an indirect relationship. • Light with a short wavelength will have a high frequency and light with a long wavelength will have a low frequency. • Light with short wavelengths has high energy and long wavelength has low energy MAP TAP 2003-2004 The Electromagnetic Spectrum 6 MAP TAP 2003-2004 The Electromagnetic Spectrum 7 Radio waves • Low energy waves with long wavelengths • Includes FM, AM, radar and TV waves • Wavelengths of 10-1m and longer • Low frequency • Used in many -

Including Far Red in an LED Lighting Spectrum
technically speaking BY ERIK RUNKLE Including Far Red in an LED Lighting Spectrum Far red (FR) is a one of the radiation (or light) wavebands larger leaves can be desired for other crops. that regulates plant growth and development. Many people We have learned that blue light (and to a smaller extent, consider FR as radiation with wavelengths between 700 and total light intensity) can influence the effects of FR. When the 800 nm, although 700 to 750 nm is, by far, the most active. intensity of blue light is high, adding FR only slightly increases By definition, FR is just outside the photosynthetically active extension growth. Therefore, the utility of including FR in an radiation (PAR) waveband, but it can directly and indirectly indoor lighting spectrum is greater under lower intensities increase growth. In addition, it can accelerate of blue light. One compelling reason to deliver at least some flowering of some crops, especially long-day plants, FR light indoors is to induce early flowering of young plants, which are those that flower when the nights are short. especially long-day plants. As we learn more about the effects of FR on plants, growers sometimes wonder, is it beneficial to include FR in a light-emitting diode (LED) spectrum? "As the DLI increases, Not surprisingly, the answer is, it depends on the application and crop. In the May 2016 issue of GPN, I wrote about the the utility of FR in effects of FR on plant growth and flowering (https:// bit.ly/2YkxHCO). Briefly, leaf size and stem length photoperiodic lighting increase as the intensity of FR increases, although the magnitude depends on the crop and other characteristics of the light environment. -

Electromagnetic Spectrum
Electromagnetic Spectrum Why do some things have colors? What makes color? Why do fast food restaurants use red lights to keep food warm? Why don’t they use green or blue light? Why do X-rays pass through the body and let us see through the body? What has the radio to do with radiation? What are the night vision devices that the army uses in night time fighting? To find the answers to these questions we have to examine the electromagnetic spectrum. FASTER THAN A SPEEDING BULLET MORE POWERFUL THAN A LOCOMOTIVE These words were used to introduce a fictional superhero named Superman. These same words can be used to help describe Electromagnetic Radiation. Electromagnetic Radiation is like a two member team racing together at incredible speeds across the vast regions of space or flying from the clutches of a tiny atom. They travel together in packages called photons. Moving along as a wave with frequency and wavelength they travel at the velocity of 186,000 miles per second (300,000,000 meters per second) in a vacuum. The photons are so tiny they cannot be seen even with powerful microscopes. If the photon encounters any charged particles along its journey it pushes and pulls them at the same frequency that the wave had when it started. The waves can circle the earth more than seven times in one second! If the waves are arranged in order of their wavelength and frequency the waves form the Electromagnetic Spectrum. They are described as electromagnetic because they are both electric and magnetic in nature. -

Resonance Enhancement of Raman Spectroscopy: Friend Or Foe?
www.spectroscopyonline.com ® Electronically reprinted from June 2013 Volume 28 Number 6 Molecular Spectroscopy Workbench Resonance Enhancement of Raman Spectroscopy: Friend or Foe? The presence of electronic transitions in the visible part of the spectrum can provide enor- mous enhancement of the Raman signals, if these electronic states are not luminescent. In some cases, the signals can increase by as much as six orders of magnitude. How much of an enhancement is possible depends on several factors, such as the width of the excited state, the proximity of the laser to that state, and the enhancement mechanism. The good part of this phenomenon is the increased sensitivity, but the downside is the nonlinearity of the signal, making it difficult to exploit for analytical purposes. Several systems exhibiting enhancement, such as carotenoids and hemeproteins, are discussed here. Fran Adar he physical basis for the Raman effect is the vibra- bound will be more easily modulated. So, because tional modulation of the electronic polarizability. electrons are more loosely bound than electrons, the T In a given molecule, the electronic distribution is polarizability of any unsaturated chemical functional determined by the atoms of the molecule and the electrons group will be larger than that of a chemically saturated that bind them together. When the molecule is exposed to group. Figure 1 shows the spectra of stearic acid (18:0) and electromagnetic radiation in the visible part of the spec- oleic acid (18:1). These two free fatty acids are both con- trum (in our case, the laser photons), its electronic dis- structed from a chain of 18 carbon atoms, in one case fully tribution will respond to the electric field of the photons. -

UNIT -1 Microwave Spectrum and Bands-Characteristics Of
UNIT -1 Microwave spectrum and bands-characteristics of microwaves-a typical microwave system. Traditional, industrial and biomedical applications of microwaves. Microwave hazards.S-matrix – significance, formulation and properties.S-matrix representation of a multi port network, S-matrix of a two port network with mismatched load. 1.1 INTRODUCTION Microwaves are electromagnetic waves (EM) with wavelengths ranging from 10cm to 1mm. The corresponding frequency range is 30Ghz (=109 Hz) to 300Ghz (=1011 Hz) . This means microwave frequencies are upto infrared and visible-light regions. The microwaves frequencies span the following three major bands at the highest end of RF spectrum. i) Ultra high frequency (UHF) 0.3 to 3 Ghz ii) Super high frequency (SHF) 3 to 30 Ghz iii) Extra high frequency (EHF) 30 to 300 Ghz Most application of microwave technology make use of frequencies in the 1 to 40 Ghz range. During world war II , microwave engineering became a very essential consideration for the development of high resolution radars capable of detecting and locating enemy planes and ships through a Narrow beam of EM energy. The common characteristics of microwave device are the negative resistance that can be used for microwave oscillation and amplification. Fig 1.1 Electromagnetic spectrum 1.2 MICROWAVE SYSTEM A microwave system normally consists of a transmitter subsystems, including a microwave oscillator, wave guides and a transmitting antenna, and a receiver subsystem that includes a receiving antenna, transmission line or wave guide, a microwave amplifier, and a receiver. Reflex Klystron, gunn diode, Traveling wave tube, and magnetron are used as a microwave sources. -

Light and the Electromagnetic Spectrum
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © JonesLight & Bartlett and Learning, LLCthe © Jones & Bartlett Learning, LLC NOTElectromagnetic FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION4 Spectrum © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALEJ AMESOR DISTRIBUTIONCLERK MAXWELL WAS BORN IN EDINBURGH, SCOTLANDNOT FOR IN 1831. SALE His ORgenius DISTRIBUTION was ap- The Milky Way seen parent early in his life, for at the age of 14 years, he published a paper in the at 10 wavelengths of Proceedings of the Royal Society of Edinburgh. One of his first major achievements the electromagnetic was the explanation for the rings of Saturn, in which he showed that they con- spectrum. Courtesy of Astrophysics Data Facility sist of small particles in orbit around the planet. In the 1860s, Maxwell began at the NASA Goddard a study of electricity© Jones and & magnetismBartlett Learning, and discovered LLC that it should be possible© Jones Space & Bartlett Flight Center. Learning, LLC to produce aNOT wave FORthat combines SALE OR electrical DISTRIBUTION and magnetic effects, a so-calledNOT FOR SALE OR DISTRIBUTION electromagnetic wave. -
19. the Fourier Transform in Optics
19. The Fourier Transform in optics What is the Fourier Transform? Anharmonic waves The spectrum of a light wave Fourier transform of an exponential The Dirac delta function The Fourier transform of ejt, cos(t) 1 f ()tF ( )expjtd Fftjtdt() ()exp 2 There’s no such thing as exp(jt) All semester long, we’ve described electromagnetic waves like this: jt Et Re Ee0 What’s wrong with this description? Well, what is its behavior after a long time? limEt ??? t In the real world, signals never last forever. We must always expect that: limEt 0 t This means that no wave can be perfectly “monochromatic”. All waves must be a superposition of different frequencies. Jean Baptiste Joseph Fourier, our hero Fourier went to Egypt with Napoleon’s army in 1798, and was made Governor of Lower Egypt. Later, he was concerned with the physics of heat and developed the Fourier series and transform to model heat-flow problems. He is also generally credited with the first discussion of the greenhouse effect as a Joseph Fourier, 1768 - 1830 source of planetary warming. “Fourier’s theorem is not only one of the most beautiful results of modern analysis, but it may be said to furnish an indispensable instrument in the treatment of nearly every recondite question in modern physics.” Lord Kelvin What do we hope to achieve with the Fourier Transform? We desire a measure of the frequencies present in a wave. This will lead to a definition of the term, the “spectrum.” Monochromatic waves have only one frequency, . Light electric field electric Light Time This light wave has many frequencies. -

Eigenvalues and Eigenvectors
Chapter 3 Eigenvalues and Eigenvectors In this chapter we begin our study of the most important, and certainly the most dominant aspect, of matrix theory. Called spectral theory, it allows us to give fundamental structure theorems for matrices and to develop power tools for comparing and computing withmatrices.Webeginwithastudy of norms on matrices. 3.1 Matrix Norms We know Mn is a vector space. It is most useful to apply a metric on this vector space. The reasons are manifold, ranging from general information of a metrized system to perturbation theory where the “smallness” of a matrix must be measured. For that reason we define metrics called matrix norms that are regular norms with one additional property pertaining to the matrix product. Definition 3.1.1. Let A Mn.Recallthatanorm, ,onanyvector space satifies the properties:∈ · (i) A 0and A =0ifandonlyifA =0 ≥ | (ii) cA = c A for c R | | ∈ (iii) A + B A + B . ≤ There is a true vector product on Mn defined by matrix multiplication. In this connection we say that the norm is submultiplicative if (iv) AB A B ≤ 95 96 CHAPTER 3. EIGENVALUES AND EIGENVECTORS In the case that the norm satifies all four properties (i) - (iv) we call it a matrix norm. · Here are a few simple consequences for matrix norms. The proofs are straightforward. Proposition 3.1.1. Let be a matrix norm on Mn, and suppose that · A Mn.Then ∈ (a) A 2 A 2, Ap A p,p=2, 3,... ≤ ≤ (b) If A2 = A then A 1 ≥ 1 I (c) If A is invertible, then A− A ≥ (d) I 1. -

User Guide Model Number RAC2V1A 802.11Ac Wave 2 Router
User Guide Model Number RAC2V1A 802.11ac Wave 2 Router C2V1A Router User Guide 1 Table of Contents 1. Overview ............................................................... 5 1.1. Introduction .................................................................................................. 5 2. Product Overview ............................................... 6 2.1. About The Router ....................................................................................... 6 2.2. What's in the Box? ..................................................................................... 6 2.3. Items You Need ........................................................................................... 6 2.4. About This Manual ...................................................................................... 7 3. System Requirements ........................................ 8 3.1. Recommended Hardware ........................................................................ 8 3.2. Windows ........................................................................................................ 8 3.3. Mac OS ........................................................................................................... 8 3.4. Linux/Unix ..................................................................................................... 8 3.5. Mobile Devices ............................................................................................. 8 4. Installing the Router ........................................... 9 4.1. Front Panel ................................................................................................... -

The Electromagnetic Spectrum CESAR’S Booklet
The electromagnetic spectrum CESAR’s Booklet The electromagnetic spectrum The colours of light You have surely seen a rainbow, and you are probably familiar with the explanation to this phenomenon: In very basic terms, sunlight is refracted as it gets through water droplets suspended in the Earth’s atmosphere. Because white light is a mixture of six (or seven) different colours, and each colour is refracted a different angle, the result is that the colours get arranged in a given order, from violet to red through blue, green, yellow and orange. We can get the same effect in a laboratory by letting light go through a prism, as shown in Figure 1. This arrangement of colours is what we call a spectrum. Figure 1: White light passing through a prism creates a rainbow. (Credit: physics.stackexchange.com) Yet the spectrum of light is not only made of the colours we see with our eyes. There are other colours that are invisible, although they can be detected with the appropriate devices. Beyond the violet, we have ultraviolet, X-rays and gamma rays. On the other extreme, beyond the red, we have infrared and radio. Although we cannot see them, we are familiar with these other types of light: for example, we use radio waves to transmit music from one station to our car receiver, ultraviolet light from the Sun makes our skin get tanned, X-rays are used by radiography machines to check if we have a broken bone, or we change channel in our TV device by sending an infrared signal to it from the remote control. -

Cannabis Grow Spectrum White Paper Growray Spectrum by Dr
Cannabis Grow Spectrum White Paper GrowRay Spectrum by Dr. Erico Mattos Plants can perceive and adapt to their environmental surroundings for optimal growth and development. Illumination is the most powerful environmental stimulus for plant growth and plants have sophisticated sensing systems to monitor light quantity, quality, direction and duration. Pigments and photoreceptors present on plants are responsible for controlling plant photosynthetic and photomorphogenic processes. The range of light capable to induce photosynthesis in plants is called “photosynthetically active radiation” (PAR) and it is defined as radiation over the spectral range of 400 – 700nm. Every absorbed photon regardless of its wavelength contributes equally to the photosynthetic process. The range of light capable of inducing photomorphogenic process ranges from 350 – 750nm. Photomorphogenic processes play a major role in developmental programs that develop new tissues. The common unit of measurement for PAR is photosynthetic photon flux density (PPFD), measured in units of moles per square meter per second. The plant’s metabolite production is also influenced by light spectrum. In addition to the primary metabolites of carbohydrates and amino‐acids, secondary metabolites are also influenced by light quality. Many secondary metabolites are key components for plants defensive mechanisms and contributors to odors, tastes and colors. At this point little is known about the manipulation of these secondary metabolites under artificial illumination systems. But what is known is that restricting the light spectrum to a handful of wavelengths can be detrimental for plant development. Using LED technology allow us to manipulate the light spectrum to trigger potential benefits in indoor cannabis production systems. In indoor cultivation systems it is possible to create a custom designed light spectrum to control plants’ development cycle, increase Favonoid and Terpene production, and enhance both biomass and THC production. -

The Electromagnetic Spectrum the Electromagnetic Spectrum the EM Spectrum Is the ENTIRE Range of EM Waves in Order of Increasing Frequency and Decreasing Wavelength
The Electromagnetic Spectrum The Electromagnetic Spectrum The EM spectrum is the ENTIRE range of EM waves in order of increasing frequency and decreasing wavelength. As you go from left right, the wavelengths get smaller and the frequencies get higher. This is an inverse relationship between wave size and frequency. (As one goes up, the other goes down.) This is because the speed of ALL EM waves is the speed of light (300,000 km/s). Things to Remember The higher the frequency, the more energy the wave has. EM waves do not require media in which to travel or move. EM waves are considered to be transverse waves because they are made of vibrating electric and magnetic fields at right angles to each other, and to the direction the waves are traveling. Inverse relationship between wave size and frequency: as wavelengths get smaller, frequencies get higher. The Waves (in order…) Radio waves: Have the longest wavelengths and the lowest frequencies; wavelengths range from 1000s of meters to .001 m Used in: RADAR, cooking food, satellite transmissions Infrared waves (heat): Have a shorter wavelength, from .001 m to 700 nm, and therefore, a higher frequency. Used for finding people in the dark and in TV remote control devices Visible light: Wavelengths range from 700 nm (red light) to 30 nm (violet light) with frequencies higher than infrared waves. These are the waves in the EM spectrum that humans can see. Visible light waves are a very small part of the EM spectrum! Visible Light Remembering the Order ROY G. BV red orange yellow green blue violet Ultraviolet Light: Wavelengths range from 400 nm to 10 nm; the frequency (and therefore the energy) is high enough with UV rays to penetrate living cells and cause them damage.