Member Newsletter
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Xref Aust Catalogue for Auction
Page:1 Website:www.mossgreen.com.au Jun 18, 2015 Lot Type Grading Description Est $A COINS & BANKNOTES - AUSTRALIA - Pre-Decimal Coins 3 $ 3d in bag (250g) and Dansco album, some 6d 1/- & Florins, 1937 Crown (3) & 1966 50c (24). (100+) 150 4 $ ES & A Bank cloth bag containing nearly 4kg of mostly KGV Pennies, the vendor's list stated to contain 1919 (33), 1920 (19) and 1925 (sighted), not checked by us for varieties, condition varied. (350+) 150 Lot 5 5 $ 1915 group with 1/- VF, 1/- 1915H EF and Florin gVF. 1,000T 6 $ B INTERNMENT CAMPS: 1915 copper 1d, gVF. 100T COINS & BANKNOTES - AUSTRALIA - Pre-Decimal Gold Lot 7 7 $ A HALF SOVEREIGN: 1915S Half-Sovereign, aUnc. 150 Lot 8 8 $ C SOVEREIGN: 1878M Sovereign, F. 300 Lot 9 9 $ A/B - 1887M Sovereign mounted to 9ct gold watch chain (29g). 600 Page:2 Website:www.mossgreen.com.au Jun 18, 2015 Lot Type Grading Description Est $A COINS & BANKNOTES - AUSTRALIA - Decimal Coins 10 $C A 2000 "The Last ANZACS" & "For Valour" PNCs, plus "Australia's First VC" $1 Unc pack and 2001 "Centenary Of The Army". (4 items) 100T 11 $ A+ Proof year sets 1978-2007 (ex 1980 1985 1990-94 1996 & 1997) plus 2001 State & Territory Proof sets and $1 Unc coin packs (8). (24 sets) 800 12 $ A 1984-2005 Unc sets, the 1984 and 1985 are in yellow plastic, a few problems in the 1966-69 issues otherwise fine. 400 13 $ A 1998-2005 Australian Baby Coin Unc sets, Cat. $1040. (8 sets) 400 14 $ A+ 1998-2005 Australian Baby Coin Proof sets, Cat $2515. -

250 Cars, 500 Drivers, Thousands of Kilometres, a Million Dollar Fundraising Target… and Just 7 Days!
250 CARS, 500 DRIVERS, THOUSANDS OF KILOMETRES, A MILLION DOLLAR FUNDRAISING TARGET… AND JUST 7 DAYS! Take a seat aboard the fifth annual Shitbox Rally and get to know the participants as they share their laughter, frustration, tears and very personal reasons for taking on the challenge Feature-length Aussie documentary THE RALLY premieres Saturday 9 May at 7.30pm on Nat Geo People WATCH THE TRAILER: http://bit.ly/TheRallyNatGeo It began as a near impossible dream and is now the largest private Cancer Council fundraiser in Australia, raising more than $4.8 million dollars and counting. After both his parents died of cancer 12 months apart, South Australia’s James Freeman decided to take action to fight the disease, creating a unique and challenging way to raise money and awareness. So began Shitbox Rally – a challenge to drive cars worth $1,000 or less across Australia via some of its most arduous dirt tracks imaginable to raise money for cancer research. In a world premiere documentary airing this May on Nat Geo People, join the fifth annual Shitbox Rally filmed in 2014 and meet the community that has been brought together by the goal to defeat cancer, many of whom have been personally touched by the disease. Armed with nothing but their shitboxes, gaffer tape and a whole lot of courage, the teams began the trip of a lifetime in Perth and headed north stopping by Meekatharra, Marble Bar and Broome before heading into The Kimberly and arriving in Darwin. The rocky canyons, sandy white beaches and breath-taking waterfalls of the Top End provide a stunning backdrop to the longest and most challenging Shitbox Rally yet. -

47. Cancer Council 7 Bridges Walk 2020
POSHPOSH PORT OUT, STARBOARD HOME Saturday 21 March 2020 The Fullerton Hotel Sydney 2020 am delighted to welcome you to the POSH Gala Ball n behalf of the entire POSH committee, thank 2020. Cancer Council NSW is committed to saving lives you to everyone who has again contributed so I and reducing the burden of cancer. Since its inception O generously to our 21st POSH gala. 21 years ago, POSH has made a phenomenal contribution to these goals, raising more than $17 million for essential I would like to warmly welcome back our dedicated, cancer research. long-time supporters, and offer an equally warm welcome to the new faces here tonight. Many of you joining us tonight are long-standing members of the POSH family. Thank you for your dedication to We are all here to enjoy an evening of fun, but more achieving a cancer free future. To those of you who are new importantly, we are also here to make a difference in the to the family, a very warm welcome to this unique event. lives of people affected by cancer. I know it is a cause that is close to many of us. Research underpins our work across every stage of the cancer journey. It provides the foundation for prevention Your enthusiastic participation in tonight’s gala will programs, which empower people to make healthier choices help Cancer Council continue its vital programs in and reduce their cancer risk; information and support for research, prevention, advocacy and support. people affected by cancer when they need it most; and an There are several ways you can get involved throughout advocacy network that ensures governments act. -

June 2019 - Page 1
Vol 28 - No. 5 email: [email protected] Circulation: 1500 A BRIGHT SEASON OF SWIMMING AHEAD The entrance to the Jamestown Swimming Pool is undergoing some changes – a colourful mural was completed at the end of May by Adelaide artist Marciano Arentez. The captivating mural adorns the once drab brown besser block walls that provided no indication of the beautiful facility behind it. Local artist and resident Helen Pammenter generously donated proceeds from her 90th Birthday Art Exhibition to the Pool Management Committee, who thought it would be fitting to put it towards a mural to brighten up the walls at the entrance. Many hands and much time went into making it all happen – from Helen’s donation to labour contributions from Brenton Arnold, Leesong Electrical, Wayne McLean Plumbing and Chris Benton. The Jamestown Swimming Pool is open from November to March and invites everyone to come and enjoy the wonderful facility behind these now beautiful walls. Story by: Vanessa Duke Pool Management Committee Helen Pammenter and Mural Artist Marciano Arentez Also in this Edition: Tacko Reunion - pg3 Sports Results & Presentations - pg18 World Record Dohne Price to Jamestown Stud - pg5 Kate’s Targa Rally Success - back page FOR SALE - 7 MARGARET STREET JAMESTOWN FOR RENT – 4 BOSTON CLOSE JAMESTOWN IMMACULATE HOME INSIDE & OUT — $175,000 4 BEDROOMS 2 BATHROOMS – $270 PER WEEK - 3 bedrooms & 1 bathroom - 4 bedrooms, main with c/f, ensuite, WIR plus BIRs & - Combined kitchen/meals with NEW elec oven c/fs to remaining 3 bedrooms - Bathroom updated (2018) - Formal lounge with floating laminate floor - NEW floor coverings & freshly painted internally - Centrally located kitchen/family/dining area featuring 70 Ayr Street Jamestown SA 5491 - NEW window treatments & light fittings s/s r/c air conditioner, elec oven/cooktop & c/f - Ducted r/c a/c as well as comb. -

Box Rallies (Shitbox Rally & Mystery Box Rally) to Fund Outstanding
Box Rallies (Shitbox Rally & Mystery Box Rally) to fund outstanding cancer research projects nationally. The National Health and Medical Research Council (NHMRC) is the pinnacle for vital funding for groundbreaking cancer research projects, but their budget only goes so far. The cancer researchers who miss out get another chance at funding via Cancer Council grants. The impact of these projects has been felt right across Australia with many of the grants made possible by Box Rallies funding. Previously, Box Rallies was able to fund multi-state research projects via Cancer Council grants. In 2019, Box Rallies & Cancer Council are taking a new approach – one that means Box Rallies will fund the most outstanding cancer research projects nationally. For the first time, Box Rallies will fund the next highest ranked grants after the NHMRC. This means that Box Rallies will fund some of the most exciting cancer research projects across the country – with the highest potential to significantly impact those affected by cancer. The grants assessed during the 2019 application process will be awarded in March 2020 with Cancer Council continuing to manage the overall funding process. Shitbox Rally and Mystery Box Rally (Box Rallies) involve over 1,450 participants driving thousands of kilometres in teams of two through remote Australian locations for days on end in cars worth under $1,000 or over 25 years old respectively. Fundraising is a big part of the accepted teams requirements and forms part of a very unique overall experience that keeps bringing people back for more. Since 2010 Box Rallies have raised over $16 million for cancer research with the 2019 goal to reach $20 million. -

September 2019 - Page 1
Vol 30 - No. 8 email: [email protected] Circulation: 1600 JAMESTOWN’S ALIVE! Photo by Rosalie Dibben of WSB Distributors Jamestown Show Fireworks There’s that feeling of excitement in the air again as Jamestown’s Oct 6th - 7th, The Jamestown Show action begins from 2pm events silly season begins all over again. Starting off this year with Sunday 6th with Stacey Pavilion entries being judged, the new the Jamestown Mural Festival happening in the main street. shearing shed in full swing, Paddock to Plate Paviion serving up great cooking ideas and flavours and live entertainment into the Sept 11th - 15th, you can watch talented artists paint large scale evening with The Borderers live @ the Roundhouse plus sheaf murals for 3 days. Meet and Greet the artists on Firday 13th Sept and boot tossing events. See our middle insert for more details. from 6pm at the RSL Combined Services Rooms for dinner @ $10 per person. Learn about the artists impressions and the Nov 2nd, Head towards the Secret Garden to find that person techniques they use. you’ve been dreaming of at the Ram and Ewe Ball during the singles hour starting at 7pm. Live entertainment from “The On Sept 21st, camp under the stars, warm your bones by the fire, Incredibles” & “Basham Brothers”, plus DJ Aikman will start from soothe your soul with live music, and fill your belly with SA craft 8pm. Tickets available online. beer and low and slow BBQ. Headliners for the event include ASH GRUNWALD, VENICE QUEENS and GRENADIERS, live at the For more information or to buy tickets to any of these events Caltowie Memorial Oval from 12.30pm for the Chilled Out n Fired please visit www.visitjamestown.com.au, scroll down the page up Music Festival. -

It's a Bumper Edition!!!
JUNE 2015 Piper THE NEWSLETTER OF THE AUSTRALIAN PIPER SOCIETY INC news PO BOX 31 ROMA 4455 INC 9880292 NSW It’s a Bumper Edition!!! Welcome to the June 2015 newslet- Winter is always the downtime for ter of the Australian Piper Society. many of us, so why not take the opportunity to plan for the warmer For the first time since I have taken weather. The APS has some fantas- up the reigns as Newsletter Editor, tic events coming up, and now is the this edition is completely made up of time to sign up. member submissions! Not only that, I had so many that I’ve had to add If you haven’t been to a PPP before, an extra EIGHT pages. So thank the upcoming event in Orange in you to everyone who has submitted September is looking like it will be a content for this newsletter. Your ripper. In January we have a flya- efforts make my life much easier. way to the Bass Strait Islands in the works. Get in quickly, it is sure to be It is important for all of us to do what popular. we can to encourage new members to society. My partner and I have The Society AGM is coming up too - added an extra member in the last see you in Bendigo in November! month, and because I’m the editor I get to put her on the front page! Safe flying all! While I don’t necessarily expect everyone to go to such extreme Scott Lewis measures to build the membership, Newsletter Editor consider introducing the society to any Piper flyers you know! DISCLAIMER Any advice contained in this newsletter has been prepared without taking into account your specific circumstances, objectives, or needs. -

Blower January 2015
THE BLOWER JANUARY 2015 Brad Shiels ready for the 2015 Bathurst 12 hour Photo supplied by Steven Shiels JANUARY 18 TH KHANACROSS “THE QUARRY” COLLEGE ROAD MT PANORAMA BATHURST LIGHT CAR CLUB 416 CONROD STRAIGHT MT PANORAMA PO BOX 444 BATHURST www.blcc.com.au Office Bearers 2014 – BLCC email: secretary [email protected] Position Name Contact No. Email President Gwyn Mulholland 0429 620 769 02 6362 0769 [email protected] Vice President Scott Sims 02 6362 9784 [email protected] Treasurer Robert Flood 0408 402 729 [email protected] Secretary Donna Sims 02 6362 9784 [email protected] Committee Matthew Windsor 0407 353 350 [email protected] Mick Tuckey 0408 659 862 [email protected] Ian Plenderleith 0438 547 375 [email protected] John Markwick 0438 443 021 [email protected] Nigel Buttriss 0419 911 608 [email protected] TBA Competition Secretary Mick Tuckey 0408 659 862 [email protected] Publicity Officer Tony Hanrahan 02 6337 1355 [email protected] Blower Editor Tony Hanrahan 02 6337 1355 [email protected] Social Director Helen Mulholland 02 6362 0769 [email protected] Property Officer TBA Historic Plates Registrar David Robinson 02 6331 7433 [email protected] Cams Delegate Helen Mulholland 02 6362 0769 [email protected] Alt. Cams Delegate David Robinson 0418 652 419 [email protected] Quarry Groundsman John Windsor Membership Officer Phil Burgett 0419 758 825 [email protected] Chaplain Doug Rowan 0427 816 616 [email protected] Website Administrator Kathy Hanrahan 0407 784 590 [email protected] Eligibility Committee Matthew Windsor 0407 353 350 [email protected] Safety / Fire Officer Scott Sims 02 6362 9784 [email protected] Patrons Cam Ashelford Arthur Davis D Moore MINUTES OF THE GENERAL MEETING OF BATHURST LIGHT CAR CLUB HELD ON WEDNESDAY 26 NOVEMBER 2014, COMMENCING AT 7.35 PM ATTENDANCE: As per attendance book. -
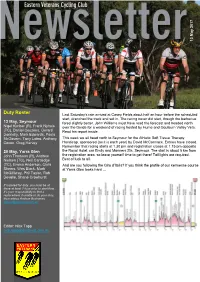
Duty Roster Last Saturday’S Rain Arrived at Casey Fields About Half an Hour Before the Scheduled Start, Drenched the Track and Set In
, 7 201 May 13 Duty Roster Last Saturday’s rain arrived at Casey Fields about half an hour before the scheduled start, drenched the track and set in. The racing never did start, though the barbecue 13 May, Seymour fared slightly better. John Williams must have read the forecast and headed north Nigel Kimber (R), Frank Nyhuis over the Divide for a weekend of racing hosted by Hume and Goulburn Valley Vets. (TC), Daniel Couzens, Gerard Read his report inside. Donnelly, Mark Edwards, Paula McGovern, Tony Lateo, Anthony This week we all head north to Seymour for the Athletic Soft Tissue Therapy Coxon, Greg Harvey Handicap, sponsored (as it is each year) by David McCormack. Entries have closed. Remember that racing starts at 1.30 pm and registration closes at 1.15 pm opposite 20 May, Yarra Glen the Royal Hotel, cnr Emily and Manners Sts, Seymour. The start is about 5 km from John Thomson (R), Andrew the registration area, so leave yourself time to get there! Tail lights are required. Nielsen (TC), Neil Cartledge Best of luck to all. (TC), Emma Anderson, Chris And are you following the Giro d’Italia? If you think the profile of our kermesse course Sheers, Wes Black, Mark at Yarra Glen looks hard … McGillivray, Phil Taylor, Rob Devolle, Shane Crowhurst If rostered for duty, you must be at there at least 1 hour prior to start time. It’s your responsibility to find a replacement if unable to do your duty, then advise Andrew Buchanan, [email protected] Editor: Nick Tapp [email protected] VVCC races, Hume Vets and Goulburn Valley Vets, 6 & 7 May How many laps of Casey before you start to feel own on the third lap hills, but figured that the 9 km that déjà vu? It helps to have the memory of a of headwind was going to bring everyone back goldfish. -

SHITBOX BREAKS FUNDRAISING RECORD 5 June 2017
MEDIA RELEASE SHITBOX BREAKS FUNDRAISING RECORD 5 June 2017 Shitbox Rally 2017 has ended with a record breaking fundraising amount – and a public marriage proposal. The rally began in Adelaide on Saturday 29 May and covered a 3,800km route to reach Cairns on Friday 2 June. The annual event raised $1.64 million for cancer research, its best result ever. As more donations come in, that tally will climb even further. Founder James Freeman said the amount included $36,500 raised by an auction and buy-back of the colourful vehicles in Cairns. He also announced the 2018 Shitbox route will be Brisbane to Darwin. “The rally teams’ fundraising effort this year is an extraordinary achievement and something we can be immensely proud of,” James said. 214 shitboxes embarked on the journey but 20 cars did not make it. In most cases, broken-down shitboxes are repaired by teams en route, but if that proves too difficult, volunteer support crew are on hand to revive the vehicles and help them re-join the rally. It was one of those support crew members, Alex Hallowell, who today proposed to girlfriend Alicia Jeffree in front of hundreds of celebrating rally members. Shitbox Rally is one of Australia’s most successful charity car rallies and this eighth rally adds to the remarkable $7.52 million it has raised since 2010. James thanked rally sponsors Bingle, Rally School, Manheim, Move Yourself and Atomix, as well as the Outback communities who warmly hosted the rally along the route. “This year’s rally included our most-ever number of unsealed roads and also took us to some incredibly beautiful, more isolated locations in Australia. -

MEDIA BACKGROUNDER: SHITBOX RALLY May 2017
MEDIA BACKGROUNDER: SHITBOX RALLY May 2017 About Box Rallies 2017 marks the eighth Shitbox Rally – this time travelling Adelaide to Cairns via the Oodnadatta Track and the Plenty Highway (27 May to 2 June). Shitbox Rally is a challenge to drive cars worth $1,000 or less across Australia via some of its toughest roads to raise money for cancer research. The rally is not a race. It’s a colourful event, attracting a loyal following of ‘Shitboxers’ – some of whom have completed the rally every year since its inception. They are brought together after experiencing cancer themselves or seeing cancer impact family and friends. It’s a chance to meet like-minded people and see Australia’s vast and beautiful country via its red dirt roads, hills and creek beds, ending each day at sports ovals and race courses and more, camping under the stars and enjoying rally friendships. To participate, each team must raise a minimum of $4,000. Shitbox Rally has raised $7.52 million since 2010 and is on track to raise $1.5 million this year. About James Freeman Founder, Shitbox Rally James Freeman founded Shitbox Rally in 2009 after both of his parents died from cancer within 12 months of each other. James also developed Mystery Box Rally and has held two Shitbox Rallies in New Zealand. The rally has become the largest independent fundraiser for Cancer Council nationally, raising more than $10 million for cancer research. While steering Box Rallies, James became a Finalist for Australian of the Year, SA in 2015. He has also been Nominated for Australian of the Year 2017. -

Sportsfields for the North at Last
THE BYRON SHIRE ~~~~~~~~~~~ ~~~~~~~~~~~~~~~~~~~~ Volume 28 #38 Tuesday, March 4, 2014 Phone 02 6684 1777 Fax 02 6684 1719 [email protected] Health & [email protected] www.echo.net.au Beauty 23,200 copies every week CAB p14–15 AUDIT A WOMAN’S PLACE IS IN THE HOUSE AND SENATE ~~~~~~~~~~~~ ~~~~~~~~~~~~ Inside Why not G20 old school Rose Wanchap Butterfl y park Who’s Beaucoup Byron Shire this psychoanalyse economics defends the for Byron? the Boz? de sport Council Notices week Fast Buck$? – p9 – p10 rocks – p11 – p13 – p19 – p36–37 Page 35 BBFF 2014 lifts off Sportsfi elds for the north at last Luis Feliu price when it fi rst went on the mar- ket was almost $2 million. Th e long saga to fi nd a suitable site Ironically, Lot 5 Shara Boulevard for sportsfi elds in the north of Byron was sold by the widow of the late Shire is over: Byron Shire Council Queensland-based developer Chum announced last Wednesday that it Vidgen, who died in 2012 aged 73 had bought a parcel of land at Shara and was oft en at odds during the Boulevard in Ocean Shores. 1990s with Council and environ- The seven-hectare block, on mentalists over his developments. the corner of Shara Boulevard and Th e site had a longtime approval Brunswick Valley Way, was bought for a service station and another, by Council for $318,000 (excluding smaller, site of 1.7 hectares just GST) and Council says it has poten- across the road, also owned by the tial for two playing fi elds, an ameni- Vigdens and up for sale, has approv- ties block, car parking, community al for a motel and restaurant.