Rush Research Resources
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
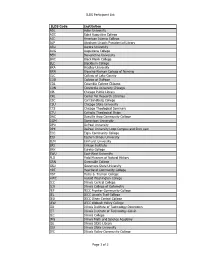
ILDS Participant List ILDS Code Institution ADL Adler University
ILDS Participant List ILDS Code Institution ADL Adler University AGC Saint Augustine College AIC American Islamic College ALP Abraham Lincoln Presidential Library ARU Aurora University AUG Augustana College BEN Benedictine University BHC Black Hawk College BLC Blackburn College BRA Bradley University BRN Blessing-Rieman College of Nursing CLC College of Lake County COD College of DuPage COL Columbia College Chicago CON Concordia University Chicago CPL Chicago Public Library CRL Center for Research Libraries CSC Carl Sandburg College CSU Chicago State University CTS Chicago Theological Seminary CTU Catholic Theological Union DAC Danville Area Community College DOM Dominican University DPU DePaul University DPX DePaul University Loop Campus and Rinn Law ECC Elgin Community College EIU Eastern Illinois University ELM Elmhurst University ERI Erikson Institute ERK Eureka College EWU East-West University FLD Field Museum of Natural History GRN Greenville College GSU Governors State University HRT Heartland Community College HST Harry S. Truman College HWC Harold Washington College ICC Illinois Central College ICO Illinois College of Optometry IEF IECC Frontier Community College IEL IECC Lincoln Trail College IEO IECC Olney Central College IEW IECC Wabash Valley College IID Illinois Institute of Technology-Downtown IIT Illinois Institute of Technology-Galvin ILC Illinois College IMS Illinois Math and Science Academy ISL Illinois State Library ISU Illinois State University IVC Illinois Valley Community College Page 1 of 3 ILDS Participant List -

Dear Illinois State Superintendent Dr. Ayala and Members of the Illinois State Board of Education
Dear Illinois State Superintendent Dr. Ayala and members of the Illinois State Board of Education, We applaud your current school reopening guidelines requiring universal masking at all times for anyone inside the school building. This mirrors what we do in clinical care to keep patients and healthcare workers safe. A group calling themselves Million Unmasked March is staging a protest in Springfield, IL, on July 25th, 2020. They are speaking out in opposition to 1) universal masking in schools and 2) COVID-19 vaccination requirements. We are aware that Million Unmasked has been approaching Illinois lawmakers asking for support, and they claim to have 6000+ supporters. As physicians, healthcare workers, parents, and citizens, we strongly disagree with their opinions on masking and vaccination. Universal masking in schools is critical to keeping students, teachers, and staff safe. As you are aware, it is a cornerstone of the containment strategies we have available to reduce viral transmission: Masking, distancing, hygiene. An international group of over 100 medical scientists endorsed universal public masking to stop the spread of COVID-19 after modeling masking’s effect on transmission rates; these models are based on an interdisciplinary review of all scientific evidence since the emergence of the novel coronavirus. Universal masking is endorsed by the Council of Medical Specialty Societies, which represents more than 800,000 doctors from 45 specialties. The American Medical Association, the American Hospital Association, and the American Nurses Association also issued a joint statement urging the public to observe universal masking. Additionally, more than 5000 concerned health care workers and citizens have signed IMPACT's petition calling for a federal mandate for masks in public. -

NMH Nursing Professional Development Pathway Overview
Northwestern Memorial Hospital Nursing Presented to: GME Program Director Presented on: January 27, 2020 Presented by: Kristin Ramsey, MSN, MPPM, RN, NE-BC Senior Vice President and Woods-Prince Family Chief Nurse Executive Northwestern Memorial Hospital An Introduction to our NMH Nurses Educational Preparation Care Delivery 96% of our clinical nurses are Bachelors prepared or higher 88% of units outperformed the NDNQI benchmark for falls 75% of eligible clinical nurses hold a specialty certification with injury and 74% outperformed this benchmark for CAUTI Nursing Research Advanced Practice Nursing 23 active research projects are being led by our clinical 365 Advanced Practice Registered Nurses nurses and are supported by NMH research consultants practice in the NM Central Region 2 An Introduction to our NMH Nurses Educational Preparation Bedside Care 93% of our clinical nurses are Bachelors prepared or higher 88% of units exceeded the NDNQI benchmark for falls 54% of eligible clinical nurses hold a specialty certification with injury and 74% exceeded this benchmark for CAUTI Nursing Research Advanced Practice Nursing 23 active research projects are being led by our clinical 365 Advanced Practice Registered Nurses nurses and supported by NMH research consultants practice in the NM Central Region 3 NMH Nurse Demographics NMH Nurse Age & Gender NMH Nurse Ethnicity Asian Black >50 15% 5% 18% Female Hispanic 91% 6% 40-50 Years <30 Years Male Unreported 13% 47% 9% 6% Caucasian Native American or 67% Pacific Islander <1% 30-40 Years 22% Identifies -

Sample of Internship Placements Phd Program in Clinical Psychology
PhD Program in Clinical Psychology Department of Psychiatry & Behavioral Sciences Sample of Internship Placements PhD Program in Clinical Psychology Year of Name and location of internship Internship Univ California-San Francisco/Clinical Psychology 2018-2019 San Francisco, CA University of Chicago Medicine 2018-2019 Chicago, IL Univ California-San Diego-Consortium/Vet Affairs 2018-2019 San Diego, CA Alpert Medical School of Brown University 2018-2019 Providence, RI Rush University Medical Center 2018-2019 Chicago, IL University of Wisconsin - Psychiatry 2018-2019 Madison, WI Children's Hospital Stanford/Children's Health 2018-2019 Palo Alto, CA University of Florida Health Science Center 2017-2018 Jacksonville, FL University of Colorado School of Medicine 2017-2018 Denver, CO Charleston Consortium Internship 2017-2018 Charleston, SC Northwestern University Feinberg School of Medicine Emory University/Grady Health System 2017-2018 Atlanta, GA Yale University - Child Study Center 2017-2018 New Haven, CT 2017-2018 Harvard Medical School/Children's Hospital Boston Advocate Illinois Masonic Medical Center 2017-2018 University of North Carolina, Chapel Hill 2016-2017 Clinical Forensic Track Chapel Hill, NC Stanford University 2016-2017 College Mental Health Track Stanford, CA Columbia University Medical Center 2016-2017 Adult track New York, NY Harvard Medical School Massachusetts General Hospital 2016-2017 Child Track Boston, MA VA Boston Healthcare System 2016-2017 General Mental Health Track Boston, MA St. Luke's Roosevelt Hospital Center 2016-2017 Adult Track New York, NY University of Chicago Medicine 2016-2017 Child Psychology track Chicago, IL UCLA - Semel Institute 2016-2017 Neuropsychology track Los Angeles, CA Jesse Brown VA Medical Center 2016-2017 General track Chicago, IL VA Boston Healthcare System 2015-2016 Neuropsychology Track Boston, MA Children’s National Medical Center 2015-2016 Washington, DC p. -

GETTING DOWN to BUSINESS Roosevelt's New President, Ali Malekzadeh, Talks About His Career and Plans for the University
FALL 2015 GETTING DOWN TO BUSINESS Roosevelt's new president, Ali Malekzadeh, talks about his career and plans for the University. PAGE 22 PAGE 52 Meet Patricia Harris, Roosevelt’s new chair of the Board of Trustees. Recommend Roosevelt to a Relative or Friend As a Roosevelt alum, you’ve accomplished a lot and are proud of your achievements. Now it’s time to encourage your son, daughter, neighbor or friend to get the same outstanding education you did. At Roosevelt University, they can have it all: Excellent academic programs Small classes Professors who care about their students An ideal location in downtown Chicago Affordable tuition and helpful financial assistance Intercollegiate athletics Countless opportunities for internships, mentorships and jobs in Chicago and the metropolitan area Roosevelt provided you with the skills you needed to succeed. Please tell a future student about the University that gave you a start. They can start by calling our Office of Admission at (877) 277-5978. FALL 2015 22 The School Year Begins During Roosevelt's Convocation on Aug. 21 in the Auditorium Theatre, new students were encouraged to send a tweet to President Ali Malekzadeh. ROOSEVELT REVIEW FALL 2015 1 fall 2015 / Volume 20, Number 2 17 Signing Off Rick Nieman (BA, '87) reminisces on his career and being one of Holland's most influential television journalists. 26 “We'll Figure It Out Together” Roosevelt honors student Desire Bernard comes from a tradition of giving. This includes regularly helping a homeless man in a Chicago park. 31 Going the Distance Alum Leo Solarte looks to make his mark on Chicago's real estate scene. -

Expanding the Boundaries of Research, Teaching and Patient Care
Expanding the boundaries of research, teaching and patient care Rush University College of Health Sciences ImpactCommunity Edition MRI Safety Day draws “Double-grads” make Bringing Occupational professionals from multiple a difference in their Therapy into the disciplines communities community Page 10 Page 20 Page 34 Contents We live the teacher - practitioner 4 Pathways to opportunity 6 Rush PA leads interdisciplinary mission to the DR model every day 8 Listening to the community’s needs 10 MRI Safety Day draws professionals from multiple disciplines 12 Helping hearts in the OR and beyond In our classrooms, clinics, labs and the communities we Buying locally to improve community wellness serve, the Rush University College of Health Sciences (CHS) 14 lives our values of collaboration and care. With more than 16 Supporting patients and families affected by Parkinson’s disease half the U.S. health care workforce in an allied health field, the need for exceptional professionals in the health sciences 18 Meet Henry, a chaplain’s best friend is constantly expanding — and our 15 programs prepare students to succeed as practitioners, managers and leaders. 20 Daring to dream The CHS is centered on Rush’s teacher-practitioner 24 Sharing a story of loss to create change model, which ensures students learn from active clinicians 26 Breathing easier who excel in their professions. We integrate didactic study, patient care, research and service in the context 28 A recipe for collaboration of a world-class medical center, and our faculty and students regularly join forces with colleagues from other 30 Testing the possibilities departments and colleges to further knowledge and improve patient outcomes. -

Academic Partnerships 1
Academic Partnerships 1 ACADEMIC PARTNERSHIPS Rush University In conjunction with the Department of Health Systems Management in DePaul University has entered into a variety of relationships with other the College of Health Sciences at Rush University Medical Center, the educational institutions to provide enhanced learning opportunities for Kellstadt Graduate School of Business of the College of Commerce offers students. a joint MBA/MS (Master of Science in Health Systems Management) degree program. American University in Paris DePaul and The American University of Paris (AUP) are partnering to Truman College, City Colleges of Chicago offer an innovative two-year program leading to an MBA from DePaul’s Through an agreement with the City Colleges of Chicago, students may Kellstadt Graduate School of Business and a M.A. in Cross-cultural and complete their first years in college at Truman College, then seamlessly Sustainable Business from AUP. transfer their credits towards a DePaul undergraduate degree through the School for New Learning. Catholic Theological Union With permission, upper-level students in Catholic Studies and Religious Wright College, City Colleges of Chicago Studies may elect to complete courses at the Catholic Theological Union. Through an agreement with the City Colleges of Chicago, students may complete their first years in college at Wright College, then seamlessly Illinois Institute of Technology transfer their credits towards a DePaul undergraduate degree through the School for New Learning. Through a five-year joint program between DePaul and the Illinois Institute of Technology, students may earn a degree in physics from DePaul and degree in engineering from IIT, with a concentration in Study Abroad Opportunities Mechanical, Aerospace, Electrical, or Computer Engineering. -
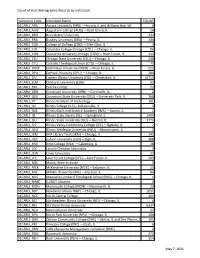
Count of Host Bibliographic Records by Institution
Count of Host Bibliographic Records by Institution Institution Code Institution Name COUNT 01CARLI_ARU Aurora University (ARU) —Aurora, IL and Williams Bay, WI 6 01CARLI_AUG Augustana College (AUG) —Rock Island, IL 16 01CARLI_BEN Benedictine University 332 01CARLI_BRA Bradley University (BRA) —Peoria, IL 144 01CARLI_COD College of DuPage (COD) —Glen Ellyn, IL 3 01CARLI_COL Columbia College Chicago (COL) —Chicago, IL 86 01CARLI_CON Concordia University Chicago (CON) —River Forest, IL 133 01CARLI_CSU Chicago State University (CSU) —Chicago, IL 348 01CARLI_CTU Catholic Theological Union (CTU) —Chicago, IL 79 01CARLI_DOM Dominican University (DOM) —River Forest, IL 212 01CARLI_DPU DePaul University (DPU) —Chicago, IL 280 01CARLI_EIU Eastern Illinois University (EIU) —Charleston, IL 16713 01CARLI_ELM Elmhurst University (ELM) 92 01CARLI_ERK Eureka College 22 01CARLI_GRN Greenville University (GRN) —Greenville, IL 2 01CARLI_GSU Governors State University (GSU) —University Park, IL 160 01CARLI_IIT Illinois Institute of Technology 191 01CARLI_ILC Illinois College (ILC)—Jacksonville, IL 3 01CARLI_IMS Illinois Math and Science Academy (IMS) —Aurora, IL 1 01CARLI_ISL Illinois State Library (ISL) —Springfield, IL 1495 01CARLI_ISU Illinois State University (ISU) —Normal, IL 3775 01CARLI_IVC Illinois Valley Community College (IVC) —Oglesby, IL 7 01CARLI_IWU Illinois Wesleyan University (IWU) —Bloomington, IL 3 01CARLI_JKM JKM Library Trust (JKM) —Chicago, IL 345 01CARLI_JUD Judson University (JUD) —Elgin, IL 388 01CARLI_KNX Knox College (KNX) —Galesburg, -

Thepractitioner
THE PRACTITIONER Summer/Fall 2019 www.rushu.rush.edu/hsm Spreading Rush Excellence In this Issue: Chair’s Report Chair’s Report 2 The healthcare landscape across the country continues to change as does our own leadership landscape within Rush. Program News 3 Since my last letter to you, Ranga Krishnan and Omar Lateef have been appointed as CEO of the Rush System for Health Faculty Update 4 and Rush University Medical Center respectively. These Faculty Spotlight 5 appointments are in addition to welcoming earlier this year a new President of Rush University, Sherine Gabriel. Changes in Research 6-7 leadership come with uncertainty but with opportunities as well for those poised to contribute. I find that as Rush continues Class profiles 8-9 to fine tune implementation of its strategy, the Department of Health Systems Management gets noted more than ever as a pivotal piece of a future Student Spotlights 10 dependent on solutions focused on the community, inter-professional collaboration, and technology to harness data and connections never thought imaginable. Our teacher Student Awards 11 practitioner model will evolve but still differentiate us in the market. It is an exciting time. Philanthropy 11-13 While our strategic options to expand and diversify are continuing, we have retained our high ranking in US News and World Report and get recognized regularly for our successes Alumni News 14-15 in other ways. In March we received the CAHME Canon Solutions American Award for Sustainability in Healthcare Education and Practice. Special thanks and congratulations go to Christopher Nolan and Shweta Ubhayakar for their efforts to make it all happen. -
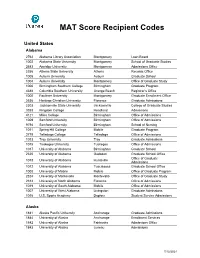
MAT Score Recipient Codes
MAT Score Recipient Codes United States Alabama 2762 Alabama Library Association Montgomery Loan Board 1002 Alabama State University Montgomery School of Graduate Studies 2683 Amridge University Montgomery Admissions Office 2356 Athens State University Athens Records Office 1005 Auburn University Auburn Graduate School 1004 Auburn University Montgomery Office of Graduate Study 1006 Birmingham Southern College Birmingham Graduate Program 4388 Columbia Southern University Orange Beach Registrar’s Office 1000 Faulkner University Montgomery Graduate Enrollment Office 2636 Heritage Christian University Florence Graduate Admissions 2303 Jacksonville State University Jacksonville College of Graduate Studies 3353 Kingdom College Headland Admissions 4121 Miles College Birmingham Office of Admissions 1009 Samford University Birmingham Office of Admissions 9794 Samford University Birmingham School of Nursing 1011 Spring Hill College Mobile Graduate Program 2718 Talladega College Talladega Office of Admissions 1013 Troy University Troy Graduate Admissions 1015 Tuskegee University Tuskegee Office of Admissions 1017 University of Alabama Birmingham Graduate School 2320 University of Alabama Gadsden Graduate School Office Office of Graduate 1018 University of Alabama Huntsville Admissions 1012 University of Alabama Tuscaloosa Graduate School Office 1008 University of Mobile Mobile Office of Graduate Program 2324 University of Montevallo Montevallo Office of Graduate Study 2312 University of North Alabama Florence Office of Admissions 1019 University -

And Mathematics
Science AND MATHEMATICS MAJORS AND MINORS SCIENCE AND MATHEMATICS MAJORS: • Biochemistry Real science, real math, taught by real Christians. At Judson University, you’ll learn how faith and fact work • Biology together to make a real difference in the world. With the variety of diverse majors, students at Judson can • Chemistry • Environmental Studies discover practical ways to address issues facing the world today in the environment, in the human body, • Mathematics in social justice issues like world hunger, and in the areas of education. • Natural Science How can you make a real difference in the world? The possibilities are endless. • Pre-Professional Programs MINORS: • Biology PROGRAM DISTINCTIVES • Chemistry • Mathematics • The Science and Math programs offer big university academics from • Science an intimate classroom setting, allowing students to experience hands-on learning and one-on-one attention from experienced faculty that still are active in their field of study. Pre-Professional Programs | Judson’s Science • The Science and Math programs work with organizations to provide and Mathematics Department offers four-year programs for research and solutions that will aid their missions while providing students who plan to apply to professional schools of medicine, students ample opportunity to share their classroom knowledge in a dentistry, chiropractic, veterinary medicine, physical therapy, or occupational therapy. The department also offers two-year real-world setting early and often. programs for students interested in careers in nursing or pharmacy. • At Judson’s World Hunger Research Center, students have After completing two years of basic science and liberal arts advanced the effort to make the velvet bean a food source for requirements at Judson, pre-nursing students can complete an emerging nations. -

View Task Force (RTF) to Review the Events Surrounding the Separation of Dr
1617-055 Wheaton Mag- Cover.indd FC2 V OLUME 20 // ISSUE 1 // 2017 CULTIVATING A DEEP LOVE FOR CHRIST AND HIS KINGDOM P. 6 and Center The Faith Neuroscience WHEATON NewArmerding theArts for The Music Hundred Holmes and 11/15/16 4:36 PM The best way to experience the invaluable education available at Wheaton is by being bold enough to ask hard questions and step beyond your comfort zone. Wheaton’s warm community and Christ-centered mission make it an excellent place to do just that.” —Peter D. ’16 As alumni and friends of Wheaton, you play a critical role in helping us identify the best and brightest students to refer to the College. We value your input and invite you to join us in the recruitment process once again. To refer a student who will take full advantage of the Wheaton Experience, please let us know at wheaton.edu/refer. To share stories from current Wheaton students and links to valuable content that will help guide prospective students as they navigate their college search journey, go to blog.wheaton.edu. 1617-055 Wheaton Mag - FOB.indd IFC1 11/22/16 9:06 AM featuresVOLUME 20 // ISSUE 1 WINTER 2017 WHEATON “All truth is God’s truth.” Facebook facebook.com/ FROM THE HEART, ART: wheatoncollege.il FOR THE KINGDOM ZACHARY ERWIN ’17 / 21 / 32 Twitter twitter.com/ wheatoncollege ➝ THE HOLMES NEUROSCIENCE HUNDRED / 30 AND FAITH / 34 Instagram courtesy of the Wheaton College Archives, Buswell Library of the Wheaton courtesy instagram.com/ Photo wheatoncollegeil WHEATON.EDU/MAGAZINE 1 1617-055 Wheaton Mag - FOB.indd 1 11/16/16 3:02 PM SECTION NAME HERE VOLUME 20 // ISSUE 1 WINTER 2017 WHEATON 2 Is Wheaton in your plans? Provide for Wheaton College’s ministry through your estate plans.