L-Ascorbic Acid (Vitamin C) (Cas No
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Electron Microscopy and Histochemical Correlation of Human Anterior Pituitary Cells
Electron Microscopy and Histochemical Correlation of Human Anterior Pituitary Cells Carlos Paiz, MD and Gordon R. Hennigar, MD THE PARS ANrERIOR of human adenohypophysis has been studied extensively by histochemical and tinctorial methods."-8 Multiple nomenclatures have been developed to describe the various cell types, but the ultimate goal is to adopt a terminology based solely on function. Electron microscopy has served to clarify in part the fine structure of the adenohypophysis, and its contribution to date has been the attempted correlation of granular size and shape with specific hormonal secretion.9-" Similar studies have been made in animal species other than man.12-'7 In several human cell types, the granules are so similar that it is fre- quently difficult if not impossible to distinguish the cells on morphologic grounds alone. The use of thick-thin section correlation for light and electron microscopy can, in part, clarify this problem, since histochem- ical properties at the light level may be correlated with fine structural differences within the same cell at the electron microscopic level. Such correlations have served three purposes: (1) We have been able to relate certain serous, mucoid, and seemingly chromophobe cells of light microscopy with the corresponding electron microscopic equiv- alents. (2) In so doing, we have demonstrated that cells having the same or similar granule morphology with electron microscopy are strik- ingly different with light microscopy histochemistry. (3) The thick-thin section method of comparison has provided us with the opportunity to relate fine structural differences within pituitary cells with the corre- sponding variable dye binding seen in a single cell with light micros- copy. -

Renal Cell Carcinoma: from a Pathologist's Perspective
SMGr up Histologic Aspect of Renal Cell Carcinomas Solène-Florence Kammerer-Jacquet and Nathalie Rioux-Leclercq* Department*Corresponding of Pathology, author: Pontchaillou Hospital, France Nathalie Rioux-Leclercq, Department of Pathology, Pontchaillou Hospital, 2 rue Henri le Guilloux, 35300 Rennes Cedex 9, France, Tel: +33 2 99 28 42 79; Fax: +Published 33 2 99 28 Date: 42 84; Email: [email protected] July 18, 2016 ABSTRACT the ISUP (International Society of Urologic Pathology). The most recent recommendations were International guidelines for the classification of renal tumors in adults are provided from (RCC). In this established in 2012, and the 2016 WHO classification incorporated these guidelines but also clinical, pathological, and molecular characteristics of the renal cell carcinomas review, we focus on the macroscopic, histologic immunohistochemical and cytogenetic criteria (ccRCC) (P-RCC) (Ch-RCC), MiT family translocation RCC, that lead to the diagnosis of RCC. The main histologic subtypes of RCC include clear cell RCC collecting duct carcinoma, and medullary renal cell carcinoma. We also describe the other and rare , papillary RCC , chromophobe RCC entities of RCC recognized in the 2016 WHO classification: hereditary leiomyomatosis associated RCC, succinate dehydrogenase deficient RCC, mucinous tubular and spindle cell carcinoma, (AML). tubulocystic RCC, acquired cystic disease associated RCC, mixed epithelial and stromal tumor of Renalthe kidney, Cell Carcinoma clear cell | www.smgebooks.com papillary RCC, and epithelioid angiomyolipoma 1 Copyright Rioux-Leclercq N.This book chapter is open access distributed under the Creative Commons Attribu- tion 4.0 International License, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited. -

Molecular Genetics and Immunohistochemistry Characterization of Uncommon and Recently Described Renal Cell Carcinomas
Review Article Molecular genetics and immunohistochemistry characterization of uncommon and recently described renal cell carcinomas Qiu Rao1*, Qiu-Yuan Xia1*, Liang Cheng2, Xiao-Jun Zhou1 1Department of Pathology, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China; 2Department of Pathology and Laboratory, Indiana University School of Medicine, Indianapolis, IN, USA *These authors contributed equally to this work. Correspondence to: Dr. Xiao-Jun Zhou. Department of Pathology, Nanjing Jinling Hospital, Nanjing University School of Medicine, Nanjing, Jiangsu 210002, China. Email: [email protected]. Abstract: Renal cell carcinoma (RCC) compromises multiple types and has been emerging dramatically over the recent several decades. Advances and consensus have been achieved targeting common RCCs, such as clear cell carcinoma, papillary RCC and chromophobe RCC. Nevertheless, little is known on the characteristics of several newly-identified RCCs, including clear cell (tubulo) papillary RCC, Xp11 translocation RCC, t(6;11) RCC, succinate dehydrogenase (SDH)-deficient RCC, acquired cystic disease- associated RCC, hereditary leiomyomatosis RCC syndrome-associated RCC, ALK translocation RCC, thyroid-like follicular RCC, tubulocystic RCC and hybrid oncocytic/chromophobe tumors (HOCT). In current review, we will collect available literature of these newly-described RCCs, analyze their clinical pathologic characteristics, discuss their morphologic and immunohistologic features, and finally summarize their molecular and genetic evidences. We expect this review would be beneficial for the understanding of RCCs, and eventually promote clinical management strategies. Keywords: Renal cell carcinoma (RCC); renal tumor; immunohistochemistry; molecular genetics Submitted Apr 14, 2015. Accepted for publication Jan 15, 2016. doi: 10.3978/j.issn.1000-9604.2016.01.03 View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2016.01.03 Introduction neoplasms and emerging/provisional new entities. -
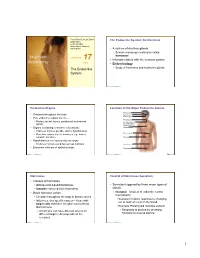
The Endocrine System
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Essentials of Abdominal Fine Needle Aspiration Cytology
1 ESSENTIALS OF ABDOMINAL FINE NEEDLE ASPIRATION CYTOLOGY Gia-Khanh Nguyen 2008 2 ESSENTIALS OF ABDOMINAL FINE NEEDLE ASPIRATION CYTOLOGY Gia-Khanh Nguyen, M.D. Professor Emeritus Laboratory Medicine and Pathology University Of Alberta Edmonton, Alberta, Canada Copyright by Gia-Khanh Nguyen Revised first edition, 2008 First edition, 2007. All rights reserved. This book was legally deposited at the Library and Archives Canada. ISNB: 0-9780929-2-9 3 TABLE OF CONTENTS Table of contents 3 Preface 4 Dedication 5 Related material 6 Key to abbreviations 7 Chapter 1. Pancreas and ampullary region 8 Chapter 2. Liver and biliary tree 39 Chapter 3. Kidney and renal pelvis 70 Chapter 4. Adrenal gland 87 Chapter 5. Other mass lesions 102 4 PREFACE The monograph “Essentials of Abdominal Fine Needle Aspiration Cytology” is written for practicing pathologists in community hospitals, residents in pathology and cytotechnologists who are interested in acquiring a basic knowledge on fine needle aspiration cytology of abdominal tumors/lesions. Commonly encountered tumors and uncommon lesions with characteristic cytologic manifestations are presented. Diagnostic criteria are presented and value and limitations of immunocytochemistry in tumor typing and differential diagnosis are stressed. For almost all lesions histopathologic images are included for cytohistologic correlation. Important references are listed in alphabetic order at the end of each chapter for further consultation. This monograph was prepared by myself. Therefore, a few typographical errors -

Corticotroph Hyperplasia and Cushing Disease: Diagnostic Features and Surgical Management
» This article has been updated from its originally published version to correct an error in the Discussion. See the corresponding erratum notice, DOI: 10.3171/2020.9.JNS201514a. « CLINICAL ARTICLE Corticotroph hyperplasia and Cushing disease: diagnostic features and surgical management Michael P. Catalino, MD, MSc,1,2 David M. Meredith, MD, PhD,3,4 Umberto De Girolami, MD,3,4 Sherwin Tavakol, MPH,1,5 Le Min, MD, PhD,6 and Edward R. Laws Jr., MD1,4 1Department of Neurosurgery, Brigham and Women’s Hospital/Harvard Medical School, Boston, Massachusetts; 2Department of Neurosurgery, University of North Carolina Hospitals, Chapel Hill, North Carolina; 3Department of Pathology, Brigham and Women’s Hospital/Harvard Medical School, Boston; 4Dana Farber Cancer Institute, Boston; 5Harvard TH Chan School of Public Health, Boston; and 6Division of Endocrinology, Brigham and Women’s Hospital/Harvard Medical School, Boston, Massachusetts OBJECTIVE This study was done to compare corticotroph hyperplasia and histopathologically proven adenomas in patients with Cushing disease by analyzing diagnostic features, surgical management, and clinical outcomes. METHODS Patients with suspected pituitary Cushing disease were included in a retrospective cohort study and were excluded if results of pathological analysis of the surgical specimen were nondiagnostic or normal. Cases were reviewed by two experienced neuropathologists. Total lesion removal was used as a dichotomized surgical variable; it was defined as an extracapsular resection (including a rim of normal gland) in patients with an adenoma, and for hyperplasia patients it was defined as removal of the presumed lesion plus a rim of surrounding normal gland. Bivariate and multivariate analyses were performed. Recurrence-free survival was compared between the two groups. -

Review Review of Sarcomatoid Renal Cell Carcinoma with Focus on Clinical
Histol Histopathol (2003) 18: 551-555 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology Review Review of sarcomatoid renal cell carcinoma with focus on clinical and pathobiological aspects N. Kuroda, M. Toi, M. Hiroi and H. Enzan First Department of Pathology, Kochi Medical School, Kohasu, Oko-cho, Nankoku City, Kochi, Japan Summary. In sarcomatoid renal cell carcinoma (RCC), Peralta-Venturina et al., 2001). In recent classifications, it is generally accepted that the sarcomatoid portion is sarcomatoid RCC is not a distinct histological entity derived from metaplastic transformation of carcinoma. because it arises from all subtypes of RCCs (Kovacs et Sarcomatoid RCCs account for about 1-8% of all renal al., 1997; Störkel et al., 1997). tumors. Macroscopically, tumors generally form encapsulated masses and show invasive growth. Epidemiology Sarcomatoid RCCs originate from all subtypes of RCCs, including conventional, papillary, chromophobe, and Sarcomatoid RCCs account for about 1-8% of all collecting duct carcinomas. With regard to the growth renal tumors (Farrow et al., 1968; Tomera et al., 1983; pattern of the sarcomatoid component, malignant fibrous Bertoni et al., 1987; Ro et al., 1987; Sella et al., 1987; histiocytomatous, fibrosarcomatous and unclassified DeLong et al., 1993; Reuter, 1993; Akhtar et al., 1997; sarcomatous patterns are frequently seen. de Peralta-Venturina et al., 2001). The mean age and Immunohistochemically, sarcomatoid RCCs are range of ages of patients were 56.2 years and 30-81 generally positive for AE1/AE3, epithelial membrane years in a large series studied by Ro et al. (1987) and 60 antigen (EMA) and vimentin and negative for desmin, years and 33-80 years in a large series studied by de actin and S-100. -

Pituitrin-Injection
Cellular changes in the anterior pituitary of the mouse following Pituitrin-injection By Masao Sano Department of Anatomy, Nagoya University School of Medicine, Nagoya, Japan. (Director : Prof. Dr. K. Y a ma da) Introduction It has been recognized that the anterior and posterior lobes of the pituitary are clearly bordered by connective tissues morphologically, and that these two lobes have not neural but humoral connection through a pituitary portal system (Popa and Fielding, '30; Wislocki and King, '36 ; 0 hf uj i, '53). On the other hand, it has also been believed that posterior pituitary hormone(s) is secreted by the pituicyte of the posterior lobe. Recently, however, B a r gmann ('49) and co-workers postulated that the hormone is produced by certain nerve cells in the hypothalamus, and that the posterior pituitary plays the role of storage and release of the hormone. Unrelated to the site of production of posterior pituitary hormone, it is presumable that the hormone acts directly on the anterior pituitary by the general blood- circulation or the portal vessels, or indirectly through the other organs. By what mechanism does the posterior pituitary hormone influence the anterior pituitary ? What histological changes are revealed in the anterior lobe then? These problems deserve much interest, but only a few studies, regarding these, have been made so far. Ito ('53) reported that an intimate relationship exists between posterior pituitary hormone and basophile cells of the anterior lobe. Also it has been shown that the hormones of the posterior pituitary and the adrenal cortex have antagonistic actions on sodium and water excretion under various experimental conditions (Winter and In gram, '43; Little et al., '47; Sartorius and Roberts, '49 and others). -

Review Review of Chromophobe Renal Cell Carcinoma with Focus
Histol Histopathol (2003) 18: 165-171 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology Review Review of chromophobe renal cell carcinoma with focus on clinical and pathobiological aspects N. Kuroda, M. Toi, M. Hiroi and H. Enzan First Department of Pathology, Kochi Medical School, Kohasu, Oko-cho, Nankoku City, Kochi, Japan Summary. In recent years, the concept of chromophobe Key words: Chromophobe renal cell carcinomas, renal cell carcinoma (RCC) has been established. Pathology, Chromosomal abnormalities Chromophobe RCCs account for about 4-6% of all renal tumors. Macroscopically, the cut surface of the tumor is generally grey-beige in color. Histologically, there are History of the establishment of the disease concept two variants (typical and eosinophilic). In the typical variant, large tumor cells with architecture of a compact Bannasch et al. (1974) described "chromophobe tubulo-cystic pattern proliferate. The cytoplasm is adenoma" as a rare form of renal tumor that was abundant and shows a fine reticular translucent pattern. experimentally induced by injection of The cell border is thick, prominent and eosinophilic. In nitrosomorpholine. Thoenes et al. (1985) found that this the eosinophilic variant, tumor cells are smaller and form is also present in human renal tumors, and they markedly eosinophilic, and a perinuclear halo is often named it "chromophobe cell renal carcinoma". They seen. Histochemically, the tumor cells generally show a later added this subtype to the classification of renal diffuse and strong reaction for Hale's colloidal iron tumors (Thoenes et al., 1986). staining. Ultrastructurally, tumor cells contain many Some investigators consider that this tumor is cytoplasmic microvesicles (150-300 nm). -

Chromophobe Renal Cell Carcinoma in 76 Years Old Female: a Case Report
Chandra A. Chromophobe renal cell carcinoma in 76 years old female: A case report. IAIM, 2017; 4(8): 139-142. Case Report Chromophobe renal cell carcinoma in 76 years old female: A case report Aviral Chandra* PG Student, Pathology Department, SBKSMI & RC, Sumandeep Vidyapeeth, Vadodara, Gujarat, India *Corresponding author email: [email protected] International Archives of Integrated Medicine, Vol. 4, Issue 8, August, 2017. Copy right © 2017, IAIM, All Rights Reserved. Available online at http://iaimjournal.com/ ISSN: 2394-0026 (P) ISSN: 2394-0034 (O) Received on: 10-07-2017 Accepted on: 22-07-2017 Source of support: Nil Conflict of interest: None declared. How to cite this article: Chandra A. Chromophobe renal cell carcinoma in 76 years old female: A case report. IAIM, 2017; 4(8): 139-142. Abstract The current World Health Organization classification of renal epithelial tumours recognises malignant lesions such as, clear cell, papillary, chromophobe and collecting duct renal cell carcinomas (RCCs), and benign entities such as oncocytoma and angiomyolipoma. Chromophobe renal cell carcinoma (RCC) is a rare variety of kidney neoplasm that represents approximately 5% of RCC. As the prognosis of chromophobe RCC depends upon early detection and typing of the RCC, meticulous histopathological examination of nephrectomy specimen is must. Key words Renal cell carcinoma, Chromophobe, Nephrectomy. Introduction consisting of chromophobe cells showing slightly Renal cell carcinoma is the most common opaque or finely reticular cytoplasm with neoplasm of the kidney [1, 2]. This malignant hematoxylin and eosin staining. Here in we are neoplasm accounts about 2-3% of all cancers. presenting a case of female patient with Chromophobe renal cell carcinoma (RCC) is a diagnosis of Chromophobe renal cell carcinoma rare variety of kidney neoplasm that represents where we are able to find and document the approximately 5% of RCC. -

Quantitative and Histomorphological Studies on Age-Correlated Changes in Canine and Porcine Hypophysis Lakshminarayana Das Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1971 Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis Lakshminarayana Das Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Animal Structures Commons, and the Veterinary Anatomy Commons Recommended Citation Das, Lakshminarayana, "Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis" (1971). Retrospective Theses and Dissertations. 4873. https://lib.dr.iastate.edu/rtd/4873 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. 71-26,847 DAS, Lakshminarayana, 1936- QUANTITATIVE AND HISTOMORPHOLOGICAL STUDIES ON AGE-CORRELATED CHANGES IN CANINE AND PORCINE HYPOPHYSIS (VOLUMES I AND II). Iowa State University, Ph.D., 1971 Anatomy• University Microfilms, A XEROX Company, Ann Arbor. Michigan Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis py Lakshminarayana Das Volume 1 of 2 A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OP PHILOSOPHY Major Subject: -

Herlant's Tetrachrome Staining, a Useful Tool for Pituitary Adenoma
Acta Medica Marisiensis 2013;59(4):209-211 DOI: 10.2478/amma-2013-0049 RESEARCH ARTICLE Herlant’s Tetrachrome Staining, a Useful Tool for Pituitary Adenoma Diagnosis Chinezu Laura1, Trouillas Jacqueline2, Loghin Andrada1, Borda Angela1 1 Department of Histology, University of Medicine and Pharmacy, Tîrgu Mureș, Romania 2 Hospices Civils de Lyon, INSERM U1028; CNRS UMR5292; Lyon Neuroscience Research Center, Neuro-oncology & Neuro-infl ammation team, University Lyon 1, Lyon, France Introduction: The morphologic diagnosis of pituitary adenomas (PA) is based on immunohistochemistry (IHC). In Romania, IHC diagnosis of PA is restricted, all of the specifi c antibodies being very expensive. A histochemical staining, Herlant’s tetrachrome (HTCS), was described several years ago, but it was not widely used for diagnostic purposes because of technical diffi culties. The aim of this paper is to bring into discussion this staining, to highlight its benefi ts, to improve the technical procedures and to establish a protocol, which combining both HTCS and IHC, facilitates the diagnosis of PA and, especially, substantially reduces the costs. Methods: HTCS was performed using normal pituitary glands. The optimal time of staining and the optimal concentration of different solutions were established for each step of the staining. Results: The improved technical procedure of HTCS is described. The staining features of all cellular types of the pituitary gland are depicted and illustrated: the chromophore cells, GH-secreting cells stained in orange, PRL-secreting cells in red-violet and ACTH-cells in dark blue, while cromophobe cells stained light blue. These staining features can be extrapolated to the diagnosis of PAs, as they consist of a prolifera- tion of such cells.