Osteoblastic/Cementoblastic and Neural Differentiation of Dental Stem Cells and Their Applications to Tissue Engineering and Regenerative Medicine
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

THE SIXTH EXTINCTION: an UNNATURAL HISTORY Copyright © 2014 by Elizabeth Kolbert
The author and publisher have provided this e-book to you for your personal use only. You may not make this e-book publicly available in any way. Copyright infringement is against the law. If you believe the copy of this e-book you are reading infringes on the author’s copyright, please notify the publisher at: us.macmillanusa.com/piracy. THE SIXTH EXTINCTION: AN UNNATURAL HISTORY Copyright © 2014 by Elizabeth Kolbert. All rights reserved. For information, address Henry Holt and Co., 175 Fifth Avenue, New York, N.Y. 10010. www.henryholt.com Jacket photograph from the National Museum of Natural History, courtesy of the Smithsonian Institution e-ISBN 978-0-8050-9979-9 First Edition: February 2014 If there is danger in the human trajectory, it is not so much in the survival of our own species as in the fulfillment of the ultimate irony of organic evolution: that in the instant of achieving self- understanding through the mind of man, life has doomed its most beautiful creations. —E. O. WILSON Centuries of centuries and only in the present do things happen. —JORGE LUIS BORGES CONTENTS Title Page Copyright Notice Copyright Epigraph Author’s Note Prologue I: The Sixth Extinction II: The Mastodon’s Molars III: The Original Penguin IV: The Luck of the Ammonites V: Welcome to the Anthropocene VI: The Sea Around Us VII: Dropping Acid VIII: The Forest and the Trees IX: Islands on Dry Land X: The New Pangaea XI: The Rhino Gets an Ultrasound XII: The Madness Gene XIII: The Thing with Feathers Acknowledgments Notes Selected Bibliography Photo/Illustration Credits Index About the Author Also by Elizabeth Kolbert AUTHOR’S NOTE Though the discourse of science is metric, most Americans think in terms of miles, acres, and degrees Fahrenheit. -

A Humble Protest a Literary Generation's Quest for The
A HUMBLE PROTEST A LITERARY GENERATION’S QUEST FOR THE HEROIC SELF, 1917 – 1930 DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jason A. Powell, M.A. * * * * * The Ohio State University 2008 Dissertation Committee: Approved by Professor Steven Conn, Adviser Professor Paula Baker Professor David Steigerwald _____________________ Adviser Professor George Cotkin History Graduate Program Copyright by Jason Powell 2008 ABSTRACT Through the life and works of novelist John Dos Passos this project reexamines the inter-war cultural phenomenon that we call the Lost Generation. The Great War had destroyed traditional models of heroism for twenties intellectuals such as Ernest Hemingway, Edmund Wilson, Malcolm Cowley, E. E. Cummings, Hart Crane, F. Scott Fitzgerald, and John Dos Passos, compelling them to create a new understanding of what I call the “heroic self.” Through a modernist, experience based, epistemology these writers deemed that the relationship between the heroic individual and the world consisted of a dialectical tension between irony and romance. The ironic interpretation, the view that the world is an antagonistic force out to suppress individual vitality, drove these intellectuals to adopt the Freudian conception of heroism as a revolt against social oppression. The Lost Generation rebelled against these pernicious forces which they believed existed in the forms of militarism, patriotism, progressivism, and absolutism. The -

VA Loggers Association News & Updates
December 2020 Issue VA Loggers Association News & Updates The "WE" Factor Producing Great Results What’s Inside WELCOME NEW MEMBERS Thank you for recently joining Supporting Members THE VIRGINIA LOGGERS ASSOCIATION Page 4 Putting our members first and proudly serving the interests of Virginia loggers! ――― President's Corner Campbell-Rodgers Lumber Co. — North Garden, VA Page 7 ――― R. A. Yancey Lumber Corp. — Crozet, VA FRA's National Outstanding Logger Low Country Timber Corp. — Snow Hill, MD. Page 8 ――― Primm Logging, Inc. (Returning Member) — Appomattox, VA Don't Take Voting January April July October For Granted Special thanks to Forestry Mutual and VLA Members for Bringing our New members! SMTWTFSSMTWTFSSMTWTFSSMTWTFS Page 10 Recruitment supports the heart & soul of our future! 1 2 3 4 1 2 3 4 ――― 1 2 3 4 1 2 3 Bring a new member today! 5 6 7 8 9 10 11 5 6 7 8 9 10 11 Director's5 6 7 Corner 8 9 10 11 4 5 6 7 8 9 10 12 13 14 15 16 17 18 12 13 14 15 16 17 18 12 13Page 14 12 15 16 17 18 11 12 13 14 15 16 17 19 20 21 22 23 24 25 19 20 21 22 23 24 25 19 20――― 21 22 23 24 25 18 19 20 21 22 23 24 26 27 28 29 30 31 26 27 28 29 30 Virginia26 27 Department 28 29 30 31 25IMPORTANT 26 27 28 29 30 31 2021 DATES of Forestry Page 15 DEC 24 Christmas Eve ――― DEC 25 Christmas Day February May August November January April July October SHARP Logger DEC 31 New Year's Eve SMTWTFSSMTWTFSSMTWTFSSMTWTFSPage 17 JAN 1, 2021 New Year's Day MerrySMTWTFSSMTWTFSSMTWTFSSMTWTFS JAN 13 Virginia General Assembly begins 1 1 2 ――― 1JAN 1 18 2 3 4Ma 5rtin Luther 6 7 King Jr. -

Experimental Sound & Radio
,!7IA2G2-hdbdaa!:t;K;k;K;k Art weiss, making and criticism have focused experimental mainly on the visual media. This book, which orig- inally appeared as a special issue of TDR/The Drama Review, explores the myriad aesthetic, cultural, and experi- editor mental possibilities of radiophony and sound art. Taking the approach that there is no single entity that constitutes “radio,” but rather a multitude of radios, the essays explore various aspects of its apparatus, practice, forms, and utopias. The approaches include historical, 0-262-73130-4 Jean Wilcox jacket design by political, popular cultural, archeological, semiotic, and feminist. Topics include the formal properties of radiophony, the disembodiment of the radiophonic voice, aesthetic implications of psychopathology, gender differences in broad- experimental sound and radio cast musical voices and in narrative radio, erotic fantasy, and radio as an http://mitpress.mit.edu Cambridge, Massachusetts 02142 Massachusetts Institute of Technology The MIT Press electronic memento mori. The book includes new pieces by Allen S. Weiss and on the origins of sound recording, by Brandon LaBelle on contemporary Japanese noise music, and by Fred Moten on the ideology and aesthetics of jazz. Allen S. Weiss is a member of the Performance Studies and Cinema Studies Faculties at New York University’s Tisch School of the Arts. TDR Books Richard Schechner, series editor experimental edited by allen s. weiss #583606 5/17/01 and edited edited by allen s. weiss Experimental Sound & Radio TDR Books Richard Schechner, series editor Puppets, Masks, and Performing Objects, edited by John Bell Experimental Sound & Radio, edited by Allen S. -

Radio 10 Gold - Top 4000
Radio 10 Gold - Top 4000 http://www.radio10gold.nl/top4000.html positie titel / artiest jaar TIME BANDITS 4000 1983 listen to the man with the golden voice MICHEL SARDOU 3999 1975 une fille aux yeux clairs NICK STRAKER BAND 3998 1999 a walk in the park CHUBBY CHECKER 3997 1965 lovely lovely NEW YORK CITY 3996 1973 i'm doing fine now BENNY NEYMAN 3995 1980 ik weet niet hoe LA BOUCHE 3994 1995 be my lover 10 CC 3993 1974 silly love BLOOD SWEAT AND TEARS 3992 1969 spinning wheel GLORIA ESTEFAN 3991 1989 here we are K-CI & JOJO 3990 1999 tell me it's real THREE DEGREES 3989 1979 the runner RICCARDO COCCIANTE 3988 1984 sincerita BRITNEY SPEARS 3987 1999 sometimes KRISTINE W 3986 1994 feel what you want BILLY STEWART 3985 1966 summertime MARSHALL AND HAIN 3984 1978 dancing in the city CLUBHOUSE 3983 1983 do it again LITTLE RICHARD 3982 1957 the girl can't help it DUSTY SPRINGFIELD 3981 1989 nothing has been proved JENNIFER LOPEZ 3980 2000 let's get loud KIM WILDE 3979 1988 never trust a stranger MICHAEL JACKSON 3978 1979 ease on down the road SPLIT ENZ 3977 1981 i hope i never ISLEY BROTHERS 3976 1969 behind a painted smile BIG COUNTRY 3975 1986 look away NELLY FURTADO 3974 2007 all good things. BELLAMY BROTHERS 3973 1976 let your love flow LIVING IN A BOX 3972 1989 room in your heart VESTA WILLIAMS 3971 1987 once bitten twice shy BARRY AND EILEEN 3970 1975 if you go OHIO PLAYERS 3969 1975 fire. -

S41404100*~41000441 Y S Oesnsarmaur NEALTM
DOCOSBIT 21112411 CS 204 043 21 151 852 Read, 'Ronald B.;Wilson, Paul I. &MOOR Coapositios Curricula. TITLE A Study of !cesim Oct 76 POB DAT/ University of Virginia OTE S4p.; Study prepared at NUS PRICE IP-30.83 MC -33.50 PlusPostage. Compositics (Literary); DESCRIPTORS College Freshmen; *Curriculum Development;Curriculum Evaluation; Curriculum leseatch;Educational Objectives; English Curricalma;English Detraction; *Evaluation Methods; nigherEducation; Models; School Surveys ABSTRACT After the historicalbackground and the contemporary evaluation are discussedis issues of freshmancomposition program sodels for compositiosinstruction are described this document, six radical, and (grammar aid composition,linguistics, literary, essay, for each model areoutlined. These editing), and tanosoaies coapositicm programs tazosonies are compared todata from a survey of providing a criticalanalysis at fourteea collegesand universities, content, schedulingprocedures, and of each progresss Although most of the progress evaluationlassessaent methods. in program emphasised the expository prosemodel, recent trends to contemporaryissues: a shift from development reflect attestios toward editing literary toward essaysodels, a shift from essay with individual models, and increasedemphasis on conferences section focuses on futureresearch and students. The concluding stads theoretical assessment needswithin the contest of this findings. Appendixesprovide a sample of framework aid preliminary used when evaluating the questionnaire and alist of geestions to be -
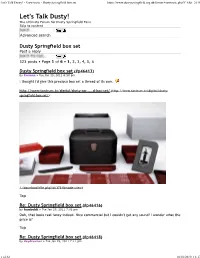
Let's Talk Dusty! 奢 View Topic
Let's Talk Dusty! • View topic - Dusty Springfield box set https://www.dustyspringfield.org.uk/forum/viewtopic.php?f=6&t=2016 Let's Talk Dusty! The Ultimate Forum for Dusty Springfield Fans Skip to content Advanced search Dusty Springfield box set Post a reply 523 posts • Page 1 of 6 • 1, 2, 3, 4, 5, 6 Dusty Springfield box set (#p46413) by Corinna » Tue Jan 25, 2011 6:50 pm I thought I'd give this precious box set a thread of its own. http://www.tantrum.tv/digital/dusty-spr ... d-box-set/ (http://www.tantrum.tv/digital/dusty- springfield-box-set/) (./download/file.php?id=3731&mode=view) Top Re: Dusty Springfield box set (#p46416) by humboldt » Tue Jan 25, 2011 7:01 pm Ooh, that looks real fancy indeed. Nice commercial but I couldn't get any sound! I wonder what the price is? Top Re: Dusty Springfield box set (#p46418) by daydreamer » Tue Jan 25, 2011 7:11 pm 1 of 32 18/10/2019, 13:17 Let's Talk Dusty! • View topic - Dusty Springfield box set https://www.dustyspringfield.org.uk/forum/viewtopic.php?f=6&t=2016 If I'm being honest, I don't actually like the packaging! I just don't think it's very Dustyish, it seems a bit dour. I would have preferred something more glamorous, maybe in the same vein as the Girl Group Sounds hatbox set. Should I ever be able to afford it, I hope the contents make me feel better about it Top Re: Dusty Springfield box set (#p46419) by Cas19 » Tue Jan 25, 2011 7:31 pm I quite like the shape of the box, and the way its all so compact. -

Logic and the Common Law Trial
University of Kentucky UKnowledge Law Faculty Scholarly Articles Law Faculty Publications Summer 1994 Logic and the Common Law Trial Richard H. Underwood University of Kentucky College of Law, [email protected] Follow this and additional works at: https://uknowledge.uky.edu/law_facpub Part of the Legal Profession Commons, and the Litigation Commons Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Richard H. Underwood, Logic and the Common Law Trial, 18 Am. J. Trial Advoc. 151 (1994). This Article is brought to you for free and open access by the Law Faculty Publications at UKnowledge. It has been accepted for inclusion in Law Faculty Scholarly Articles by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Logic and the Common Law Trial Notes/Citation Information American Journal of Trial Advocacy, Vol. 18, No. 1 (Summer 1994), pp. 151-199 This article is available at UKnowledge: https://uknowledge.uky.edu/law_facpub/261 Logic and the Common Law Trial Richard H. Underwoodt The only certain ground for discovering truth is the faculty of discriminat- ing false from true.... Otherwise, I can assure you, you will be led by the nose by anyone who chooses to do it, and you will run after anything they hold out to you, as cattle do after a green bough.' I. Introduction Throughout history, right up to the present day, there have been persistent, if somewhat contradictory, attempts by members of the legal guild to attribute to the practice of law some of the characteristics of religion and science.' Fortunately, references to the trial lawyer as "the priest in the [T]emple of [J]ustice" 3 have pretty much disappeared from the literature. -

The Fifth Commandment: a Biography of Shapurji Saklatvala and Memoir by His Daughter by Sehri Saklatvala First Digital Edition, July 2012
The Fifth Commandment: A Biography of Shapurji Saklatvala and Memoir by his Daughter By Sehri Saklatvala First digital edition, July 2012. Originally published by Miranda Press, July 1991, with ISBNs 0951827405 & 978-0951827406. This work is licensed under the Creative Commons Attribution-NonCommercial- NoDerivs 3.0 Unported License. 2 Table of Contents Editor's Note 5 Author's Preface 6 Chapter 1 - The Sun Rises in the East 8 Chapter 2 - The Plague Years 20 Chapter 3 - The Quest for Iron 31 Chapter 4 - The Sun Veers to the West 41 Chapter 5 - The Quest for a Political Solution 54 Chapter 6 - The Mind is its Own Place 66 Chapter 7 - Freedom for Me and Mine, Bondage for Thee and Thine 88 Appendix A to Chapter 7: Statement of the Workers’ Welfare League of India, 1919 112 Appendix B to Chapter 7: ‘The Call of the Third International’ 123 Appendix C to Chapter 7: Terms of Comintern Membership 129 Chapter 8 - A Communist in Parliament 135 Appendix A to Chapter 8: Report to the Labour Party Conference, 1922 147 Appendix B to Chapter 8: ‘Explanatory Notes on the Third International’ 151 Appendix C to Chapter 8: Saklatvala’s Election Addresses of 1922 159 Chapter 9 - A New Voice for the People 167 Chapter 10 - Speaking Against Imperialism 201 Chapter 11 - The Deportations to Ireland 212 Chapter 12 - The MP for Battersea and India 240 Chapter 13 - A Narrow Defeat 261 Chapter 14 - Re-election and the Red Scare 274 Chapter 15 - Banned from the USA 296 Chapter 16 - A Subversive in Parliament 314 Chapter 17 - The General Strike and a Term of Imprisonment -

(Writers) Titles Az out Now!
TITLES A-Z (WRITERS) IF YOU DON'T KNOW ME OUT NOW! wea BY NOW TY THE NEW SINGLE YZ 377/T/CD ORDER FROM3™!luea UTEO BY TELE-ORDER uea RECORDS DESK ONLTD. 01-998 OA 5929 WARNER OR FROM COMMUNICATIONS YOUR LUBa SALESPERSON. CO. ^^T* I.W7-I.21 Numb.rlD.ilntol jWll SHE'S A MYSTERY TO ME virgin vsm 1173 (E)) 9 Arfisls (Ptcw'uccfij PyWrshcri (IllfY 38 cm Roy Orhison (Bono) Blue Mountain Music ■ 3 SSl7Pa,ficLUonord,W0rn.aoppeK^^ 39 « p DustyNOTHING Springfield HAS BEEN(Pet Shop PROVED Boys/Julian Mendelsohn)PudopKoni.|12|R6207(E) Cage/10 ® 2 2 5t JosonTOO MANYDonovon BROKEN (Sfock/Aitken/Wolerman] HEARTS 0 All Boys MusicPWlPW«n32(P| ® 40 2 2 OF COURSE I'M LYING Mnrcury/Phonogrom YELLO 3(12) (F) A 6, THIS TIME 1 KNOW IT'S FOR REAL 0 Won...Brotk.<sU7780(T)|W) YelloLOVE (Yeilo) IN THE Warner NATURAL Chappell WAY Music MaKiM(Dlllf| 3 3 5 Donna Summer (Slock/Ailken/Waterraan) All Boys Music/EMI Music ® 41 32 5 Kim Wilde (Ricki Wilde/Tony Swain) Rickim Publishing ® A 4 ^ t STRAIGHTPaulo Abdul UP (Elliot WolH/Keith Cohen) Virgin MusicSiren/Virgin ® SRNIDI11 (E) 42 30 10 FourINDESTRUCTIBLE Tops (Bobby Sandstrom) Jobete MusicArista ® 112074112--612074) (BMG) A 3, KEEPONMOVIN' 10/Vir9inTEN|l(j263{E| TURNUPTHEBASS Ha/londonFFR(X| 24(F) 5 3 3 Soul II Soul leal. Coron Wheeler (Jozile B/Nellie Hooper) Virgin ® 43 23 0 Tyree feat. Kool Rock Steady (Tyree) EMI Music 6 » , GunsPARADISE N' Roses CITY (Mike Clink) Warner Chappell MusicGe(fenGEF(T)50(W) ® i 44 3, 5 VERONICAElvis Costello {Coslello/Killen/Bumetl} MPL Com./PlangentWarner Brothers W Vision 7558(T] (W) 7 „8 GloriaCAN'T EslelanSTAY AWAY & Miami FROM Sound YOU Machine (Emilio/TheEpici51<M47(6514«81(C| Jerks) SBK ® | 45 28 5 WAGES DAY CBSDEAC(T)8(C| ,o 6, I'D RATHER JACK PWLPWL(T)25|P1 Deacon Blue (Warne Livsey) ATV Music ® A 8 The Reynolds Girls (Slock/Aitken/Watcrman) All Boys Music ® i 46 1 2 Ellis,BIG BUBBLES,Beggs & Howard NO TROUBLES (Ralph P. -
The Archives of Let's Talk Dusty! - In…
2010-07-27 The Archives of Let's Talk Dusty! - In… The Archives of Let's Talk Dusty! Home | Profile | Active Topics | Active Polls | Members | Search | FAQ Username: Password: Login Save Password Forgot your Password? All Forums Let's Talk Dusty! The Forum Forum Locked You Set My Dreams To Music Printer Friendly In Private Author Topic MissDustyFanatic Posted - 06/01/2009 : 06:29:50 Where am I going? I've always loved this song, from the first listen I had of it on Simply...Dusty in 2005. Just a fun, upbeat, catchy song, and perfect for her voc al range at the time. I know it reac hed #14 in the UK in 1989, and was never released in the US until The Dusty Springfield Anthology box set in 1997. This has to be my favorite song from the Reputation album. I have to admit, though, I'm a bit baffled. USA 2606 Posts WHY was the song never released in the US? After the success of 'What Have I Done to Deserve This?', I think it could have been a sizeable hit on the Billboard c harts and gained Miss Dusty a whole new fanbase who was listening to this kind of music at the time. EMI/Parlophone really missed the boat on that one. WHY did the song only ever reach #14 in Britain? It's one of her strongest, most c ommerc ial singles, and I would think that it should have charted a lot higher, maybe top 5. And as a bonus, here's the video from Youtube http://www.youtube.com/watch?v=2bi2mdrbXL4 Feel free to comment on the song, discuss the lyrics, share you thoughts about it.. -

Christina School District Assignment Board Grade Level
Christina School District Assignment Board Grade Level: 10th Week of May 4th, 2020 Day 1 Day 2 Day 3 Day 4 Day 5 ELA This week we will Read the text, Re-read the text. Read the two Finish the story. use all that you “Escape from Answer the poems “Alcatraz” Using details from have learned Alcatraz”. Pay Digging Deeper and “Blue Prints the article, write a throughout the close attention to Questions 1-7 of Escape”. short story about school year and how the author Answer the the escape from your creative side describes the questions that Alcatraz from the to deepen your prison. Underline follow. point of view of understanding of words/phrases that Frank Morris or one literary concepts support an escape of the Anglin using the text proof prison. brothers. Use “Escape from After reading, descriptive Alcatraz”. respond briefly; language, include a ------------------------ Alcatraz was specific setting, Brainstorm. What considered escape- and dialogue. Be makes a place proof. Do you sure to include their escape proof? agree or disagree? fate—that is, Write a paragraph Why/why not? whether they or compile a list. If survived or not. you were confined to a place what items or procedures would make that place impossible to escape from? Does it have a unique location? Are there guards/machines, Christina School District Assignment Board or other things that would make this place hard to escape? Is escape proof even a realistic concept? Math Law of Cosines Complete Law of Read pages 69-70. Complete p. 72 #11- Complete p. 72 #25- (IM2) Cosines Worksheet 2 (attached) Use the 24.