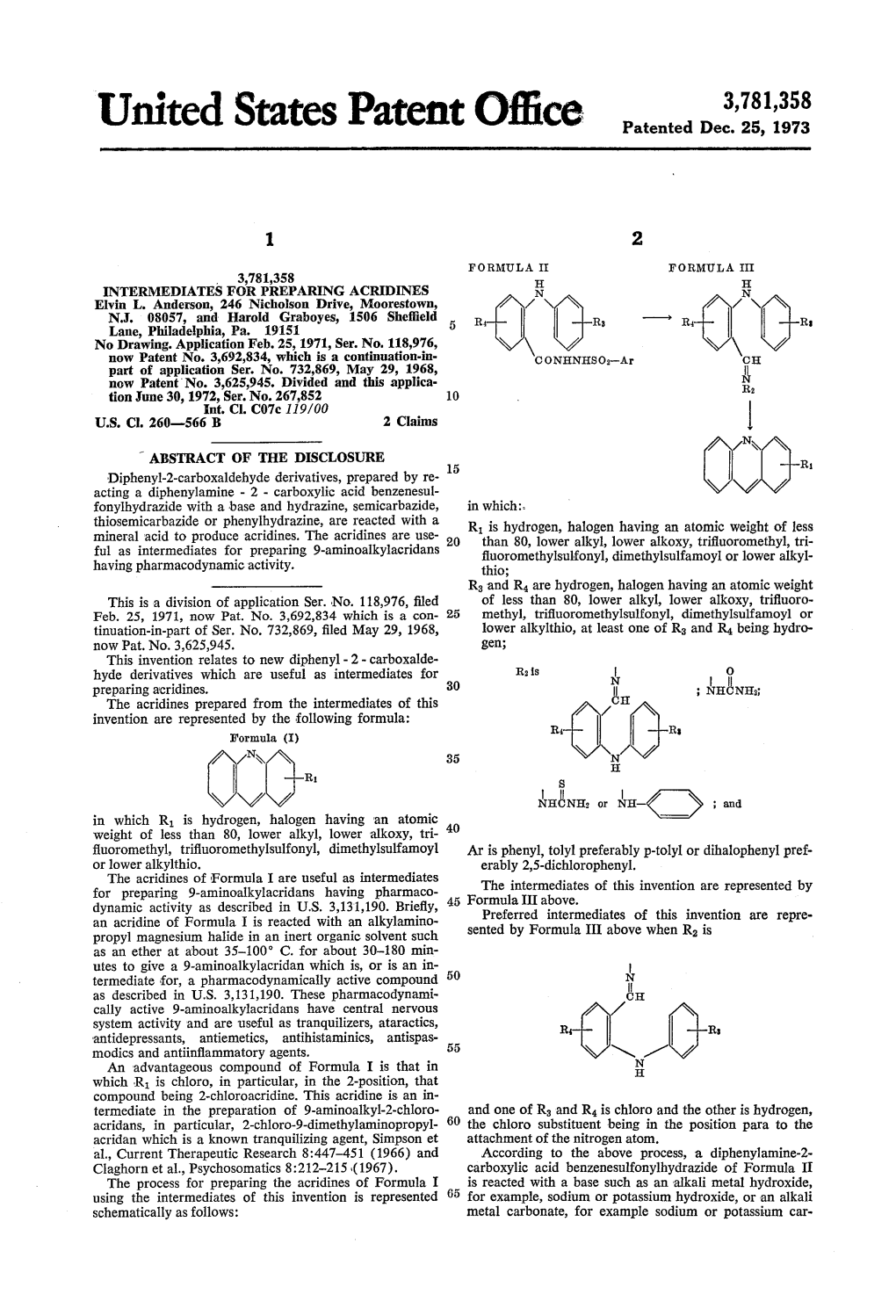
United States Patent Office Patented Dec
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monoamine Oxydases Et Athérosclérose : Signalisation Mitogène Et Études in Vivo
UNIVERSITE TOULOUSE III - PAUL SABATIER Sciences THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE TOULOUSE III Discipline : Innovation Pharmacologique Présentée et soutenue par : Christelle Coatrieux le 08 octobre 2007 Monoamine oxydases et athérosclérose : signalisation mitogène et études in vivo Jury Monsieur Luc Rochette Rapporteur Professeur, Université de Bourgogne, Dijon Monsieur Ramaroson Andriantsitohaina Rapporteur Directeur de Recherche, INSERM, Angers Monsieur Philippe Valet Président Professeur, Université Paul Sabatier, Toulouse III Madame Nathalie Augé Examinateur Chargé de Recherche, INSERM Monsieur Angelo Parini Directeur de Thèse Professeur, Université Paul Sabatier, Toulouse III INSERM, U858, équipes 6/10, Institut Louis Bugnard, CHU Rangueil, Toulouse Résumé Les espèces réactives de l’oxygène (EROs) sont impliquées dans l’activation de nombreuses voies de signalisation cellulaires, conduisant à différentes réponses comme la prolifération. Les EROs, à cause du stress oxydant qu’elles génèrent, sont impliquées dans de nombreuses pathologies, notamment l’athérosclérose. Les monoamine oxydases (MAOs) sont deux flavoenzymes responsables de la dégradation des catécholamines et des amines biogènes comme la sérotonine ; elles sont une source importante d’EROs. Il a été montré qu’elles peuvent être impliquées dans la prolifération cellulaire ou l’apoptose du fait du stress oxydant qu’elles génèrent. Ce travail de thèse a montré que la MAO-A, en dégradant son substrat (sérotonine ou tyramine), active une voie de signalisation mitogène particulière : la voie métalloprotéase- 2/sphingolipides (MMP2/sphingolipides), et contribue à la prolifération de cellules musculaire lisses vasculaires induite par ces monoamines. De plus, une étude complémentaire a confirmé l’importance des EROs comme stimulus mitogène (utilisation de peroxyde d’hydrogène exogène), et a décrit plus spécifiquement les étapes en amont de l’activation de MMP2, ainsi que l’activation par la MMP2 de la sphingomyélinase neutre (première enzyme de la cascade des sphingolipides). -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Silybum Marianum (Milk Thistle) Flower in Vitro and on Human Explants
Molecules 2015, 20, 3549-3564; doi:10.3390/molecules20033549 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Anti-Glycation Activities of Phenolic Constituents from Silybum marianum (Milk Thistle) Flower in Vitro and on Human Explants Seoungwoo Shin, Jung-A Lee, Minkyung Kim, Hyunwoo Kum, Eunsun Jung * and Deokhoon Park * Biospectrum Life Science Institute, Eines Platz 11th FL, 442-13 Sangdaewon Dong, Seoungnam City, Gyunggi Do 462-807, Korea; E-Mails: [email protected] (S.S.); [email protected] (J.-A.L.); [email protected] (M.K.); [email protected] (H.K.) * Authors to whom correspondence should be addressed; E-Mails: [email protected] (E.J.); [email protected] (D.P.); Tel.: +82-31-750-9400 (E.J. & D.P.); Fax: +82-31-750-9494 (E.J. & D.P.). Academic Editor: Derek J. McPhee Received: 25 November 2014 / Accepted: 15 February 2015 / Published: 19 February 2015 Abstract: Glycation is an ageing reaction of naturally occurring sugars with dermal proteins, with clinical signs appearing in vivo around age 30, and increasing steadily/regularly with age. The suppleness of the dermis is affected by the formation of bridges between proteins and sugars (Maillard’s reaction). The accumulation of advanced glycation end products (AGEs) in skin plays a very important role in skin ageing. Therefore, natural compounds or extracts that possess antiglycation activities may have great anti-ageing potential. In the present study, Silybum marianum flower extract (SMFE) was demonstrated to possess antiglycation activity. We found that SMFE inhibits glycation reaction between BSA and glucose. In addition, antiglycation activity of SMFE was confirmed in a human skin explants model. -

Toxicological Profile for Hydrazines. US Department Of
TOXICOLOGICAL PROFILE FOR HYDRAZINES U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry September 1997 HYDRAZINES ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. HYDRAZINES iii UPDATE STATEMENT Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 HYDRAZINES vii CONTRIBUTORS CHEMICAL MANAGER(S)/AUTHOR(S): Gangadhar Choudhary, Ph.D. ATSDR, Division of Toxicology, Atlanta, GA Hugh IIansen, Ph.D. ATSDR, Division of Toxicology, Atlanta, GA Steve Donkin, Ph.D. Sciences International, Inc., Alexandria, VA Mr. Christopher Kirman Life Systems, Inc., Cleveland, OH THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1 . Green Border Review. Green Border review assures the consistency with ATSDR policy. 2 . Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying end points. 3. Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. HYDRAZINES ix PEER REVIEW A peer review panel was assembled for hydrazines. The panel consisted of the following members: 1. Dr. -

Synthetic and Naturally Occurring Hydrazines As Possible Cancer Causative Agents
[CANCER RESEARCH 35, 3693-3697 December 1975] Synthetic and Naturally Occurring Hydrazines as Possible Cancer Causative Agents Bela Toth' The Eppley Institute for Research in Cancer, University of Nebraska Medical Center, Omaha, Nebraska 68105 SUMMARY SYNTHETIC HYDRAZINES The various synthetic substituted hydrazines, which cause tumors in animals, are briefly enumerated. To date, 19 of Studies on the carcinogenic potentialities of synthetic them have proved to be tumorigenic in animals. A number substituted hydrazines began in 1962, when it was shown of these chemicals are found today in the environment, in that the base compound hydrazine sulfate induced lung industry, in agriculture, and in medicine, and the human neoplasms in mice (1). Subsequently, a series of hydrazine population is exposed to a certain degree to some of them. derivatives were investigated in various laboratories for Hydrazine also occurs in nature in tobacco and tobacco tumor-inducing capabilities. These studies clearly demon smoke. The three other naturally occurring hydrazine strated that these chemicals are indeed powerful tumori compounds are N-methyl-N-formylhydrazine, which oc genic substances in mice, hamsters, and rats, due to their curs in the wild edible mushroom, Gyromitra esculenta, tumor-inducing abilities in the intestines, brain, lungs, and @-N-[―y-L(+)-glutamylJ-4-hydroxymethylphenyl blood vessels, liver, breasts, kidneys, etc. Now, we know of hydrazine and 4-hydroxymethylphenylhydrazine, whkh 19 hydrazine derivatives that have been shown to be tumor are found in the commonly eaten cultivated mushroom, inducers. These include, in addition to hydrazine (1, 32), Agaricus bisporus. Tumorigenesis studies with the natu methyl- (35, 40), 1,2-dimethyl- (6, 27, 36, 46, 52), 1,1- rally occurring hydrazines are in progress. -

Receptor Antagonist
Europäisches Patentamt *EP001604983A1* (19) European Patent Office Office européen des brevets (11) EP 1 604 983 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: C07D 217/26, C07D 403/06, 14.12.2005 Bulletin 2005/50 C07D 403/12, C07D 491/113, A61K 31/472, A61K 31/4741, (21) Application number: 04721010.9 A61P 43/00, A61P 29/00, (22) Date of filing: 16.03.2004 A61P 35/00, A61P 15/00, A61P 15/08, A61P 15/10, A61P 15/14, A61P 37/00, A61P 5/48, A61P 3/10, A61P 3/02, A61P 3/06, A61P 17/00, A61P 19/02, A61P 19/08 (86) International application number: PCT/JP2004/003496 (87) International publication number: WO 2004/083184 (30.09.2004 Gazette 2004/40) (84) Designated Contracting States: • HINUMA, Shuji AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Tsukuba-shi, Ibaraki 3050821 (JP) HU IE IT LI LU MC NL PL PT RO SE SI SK TR • KANZAKI, Naoyuki, c/o Takeda Pharmaceutical Designated Extension States: Yodogawa-ku, Osaka-shi, Osaka (JP) AL LT LV MK • BANNO, Yoshihiro, c/o Takeda Pharmaceutical Yodogawa-ku, Osaka-shi, Osaka (JP) (30) Priority: 17.03.2003 JP 2003072709 • YOSHIDA, Hiromi Yuki-gun, Ibar aki 3002741 (JP) (71) Applicant: Takeda Pharmaceutical Company • MATSUMOTO, Hirokazu Limited Tsukuba-shi, Ibaraki 3050821 (JP) Osaka 541-0045 (JP) (74) Representative: Rickard, Timothy Mark Adrian (72) Inventors: Takeda Euro IP Department, • ITOH, Fumio 11-12 Charles II Street Tsukuba-shi, Ibaraki 3050821 (JP) London SW1Y 4QU (GB) (54) RECEPTOR ANTAGONIST (57) A compound represented by -

1-Alkyl--1-Phenylhydraz Ines: Their Synthesis And
1-ALKYL--1-PHENYLHYDRAZ INES: THEIR SYNTHESIS AND OXIDATIVE REARRANGEMENT LACELAN MACLEAN B.Sc. Ph.D. Chemistry UNIVERSITY OF EDINBURGH 1977 To Irod parents ACKNOWLEDGEMENTS I would like to express my gratitude to Dr. A.J. Bellamy for his advice, encouragement. and assistance throughout the duration of this study. I also thank the staff and fellow students of the Chemistry Department for their general helpfulness: in particular Dr. E. Stefaniuk and Mr. C.D. Anderson for supplying samples of 2-forxnylstilbene and pent-3-en-2--one respectively, and Mrs. J. Gorrie for her care in typing this thesis. Finally, I thank the Science Research Council for financial support. L.M. I have composed this thesis which describes my own work. Where the work of other authors is referred to, this is clearly indicated. ABSTRACT OF THESIS Some historical aspects of the oxidation reactions of 1,1-disub- stituted hydrazines are reviewed. In particular, the evidence for the proposed intermediacy of diazenes in these reactions is outlined and the nature of the oxidation products is considered in the broader context of diazene-generating reactions. The synthesis of l-alkyl-l-phenylhydrazines has been examined and several preparative procedures are discussed. The alkylation of the sodium salt of phenyihydrazine with the appropriate alkyl halides was found to be the most successful method; the hydrazines prepared in this way all had substituents of the allylic type. Purification (to remove residual phenyihydrazine) can be achieved by selective recrystallisation of the hydrochloride derivative from benzene. Some limitations of this general method are described. The oxidation studies were carried out using lead tetra-acetate and mercuric oxide. -

HAZARD ASSESSMENT REPORT Hydrazine
HAZARD ASSESSMENT REPORT Hydrazine CAS No. 302-01-2 Chemicals Evaluation and Research Institute (CERI), Japan This report was prepared by CERI in collaboration with National Institute of Technology and Evaluation (NITE) under the sponsorship of New Energy and Industrial Technology Development Organization (NEDO). Preface to the English Version of the Hazard Assessment Reports For six years from April 2001 to March 2007, Chemicals Evaluation and Research Institute (CERI/Japan) was engaged in a project named “Chemical Risk Assessment and Development of Risk Assessment Methods” under "Comprehensive Chemical Substance Assessment and Management Program" funded by New Energy and Industrial Technology Development Organization (NEDO/Japan). Under this project, about 150 chemical substances were selected among those designated as Class-I Chemicals in the Law for Pollutant Release and Transfer Register and Promotion of Chemical Management (hereafter PRTR Law)1). The selection criteria of these chemicals were their priorities for risk assessment based on their production levels and environmental/human health concerns. CERI developed the hazard assessment reports of these selected chemical substances based on the review and evaluation of the environmental and human health hazard data obtained from the existing evaluation documents released by the regulatory agencies and international organizations as well as those from the published scientific literatures. The data review and compilation of the reports were conducted according to the guidelines2) and the guidance manual2) developed for this project. The proposed hazard assessment reports by CERI were reviewed by the experts in the relevant scientific fields from both inside and outside this project for accuracy, relevance and completeness. The final reports were published in Japanese after going through the deliberation by the “Council on Chemical Substances” under the Ministry of Economy, Trade and Industry (METI/Japan), which is responsible for regulation of chemical substances in Japan. -

DMA-Damaging Activity in Vivo and Bacterial Mutagenicity of Sixteen Hydrazine Derivatives As Related Quantitatively to Their Carcinogenicity1
[CANCER RESEARCH 41, 1469-1482, April 1981) 0008-5472/81 /0041-OOOOS02.00 DMA-damaging Activity in Vivo and Bacterial Mutagenicity of Sixteen Hydrazine Derivatives as Related Quantitatively to their Carcinogenicity1 Silvio Parodi, Silvio De Flora, Marco Cavanna, Albiana Pino, Luigi Robbiano, Carlo Bennicelli, and Giovanni Brambilla2 Departments of Pharmacology [M. C.. A. P., L. P., G. B.¡,Hygiene [S. D. F., C. B.]. and Oncology [S. P.¡,Genoa University. 16132 Genoa, Italy ABSTRACT enees existing in the carcinogenic potency of different chemi cals, qualitative statements of positivity or negativity in a short- Sixteen hydrazine derivatives (hydrazine, 1,1-dimethylhydra- term test are of very limited value for an assessment of the zine, 1,2-dimethylhydrazine, phenylhydrazine, procarbazine, carcinogenic risk. isoniazid, isocarboxazid, nialamide, 2,4-dinitrophenylhydra- In this work, we attempted a quantitative comparison of the zine, phenelzine, hydralazine, dihydralazine, carbamylhydra- potencies of 16 hydrazine derivatives in: (a) inducing DNA zine, mebanazine, iproniazid, and 1-carbamyl-2-phenylhydra- fragmentation in liver and lung of male Swiss mice, as evaluated zine) were tested for DNA-damaging activity by the alkaline by alkaline elution technique; (b) inducing mutations in the elution technique and for mutagenic activity in the Salmonella- Sa/mone/te-microsome test, both in the presence and in the microsome (Ames) test. The first nine compounds listed (56%) absence of S-9 mix; and (c) inducing pulmonary tumors in were found to induce a significant DNA fragmentation in the chronically treated mice. The significance of a quantitative liver and/or in the lung of i.p.-treated male Swiss mice. -

Synthesis of Insecticidal Mono- and Diacylhydrazines for Disruption of K+ Voltage-Gated Channels, Elucidation of Regiochemistry, And
Synthesis of Insecticidal Mono- and Diacylhydrazines for Disruption of K+ Voltage-Gated Channels, Elucidation of Regiochemistry, and Description of Conformational Isomerism by NMR Spectroscopy and Computation Joseph Shelby Clements II Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Chemistry Paul R. Carlier, Chair David G. I. Kingston James M. Tanko Gordon T. Yee May 2, 2017 Blacksburg, VA Keywords: Hydrazine, acylation, α-effect, regioselectivity, hydrazide, voltage- gated, (E)-/(Z)-, 19F NMR, single electron transfer (SET), rotational barrier, N-N bond rotation, rotational isomerism, helicity, transition state, dihedral angle Copyright 2017, Joseph S. Clements II Synthesis of Insecticidal Mono- and Diacylhydrazines for Disruption of K+ Voltage-Gated Channels, Elucidation of Regiochemistry, and Description of Conformational Isomerism by NMR Spectroscopy and Computation Joseph Shelby Clements II ABSTRACT Based on the success of diacyl-tert-butylhydrazines RH-5849 and RH-1266 in controlling agricultural crop pests, we endeavored to synthesize our own diacylbenzyl- and arylhydrazine derivatives for use against the malaria vector Anopheles gambiae. In the process of producing a library of compounds for assay against An. gambiae, it became clear that employing regioselective acylation techniques (in molecules that feature two nucleophilic, acyclic nitrogen atoms α to one another) would be imperative. Synthesis of the library derivatives proceeded rapidly and after topical assay, we found three compounds that were more toxic than the RH- series leads. One of the three displayed an LD50 value of half that of RH-1266, though patch clamp assay concluded that toxicity was not necessarily linked to inhibition of mosquito K+ channel Kv2.1. -

Simultaneous Determination of Phenylhydrazine, Hydrazine and Sulfite Using a Modified Carbon Nanotube Paste Electrode
Int. J. Electrochem. Sci., 5 (2010) 1203 - 1212 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Simultaneous Determination of Phenylhydrazine, Hydrazine and Sulfite Using a Modified Carbon Nanotube Paste Electrode Nahid Rastakhiz 1, Ashraf Kariminik 1, Vahhab Soltani-Nejad 2,* , Sara Roodsaz 2 1 Islamic Azad University , Kerman Branch, Iran 2 Department of Chemistry, Islamic Azad University , North Tehran Branch, Tehran , Iran *E-mail: [email protected] Received: 19 July 2010 / Accepted: 30 July 2010 / Published: 1 September 2010 A sensitive and selective electrochemical method for the determination of phenylhydrazine (PH) was developed using a modified carbon nanotube paste electrode. It has been found that under the optimum condition (pH 5.0) in cyclic voltammetry, the oxidation of PH occurred at a potential of about 250 mV on the surface of the modified electrode. The linear range of 0.6 to 900.0 µM and a detection limit of 1.31 × 10 -7 M were observed for PH in optimum conditions. The modified electrode showed an excellent character for electrocatalytic oxidization of PH, hydrazine (HZ) and sulfite (ST). Finally, this method was also examined as a selective, simple and precise electrochemical sensor for the determination of PH, HZ and ST in real samples such as urine and water samples. Keywords: Phenylhydrazine, hydrazine, sulfite, carbon nanotube paste electrode, voltammetry 1. INTRODUCTION Carbon paste electrode (CPE) is a special kind of heterogeneous carbon electrode consisting of mixture prepared from carbon powder (as graphite, glassy carbon and others carbonaceous materials) and a suitable water-immiscible or non-conducting binder [1-3]. The use of carbon paste as an electrode was initially reported by Adams [4]. -

The Action of Semicarbazide Hydrochloride on the P-Quinones 1
The Action of Semicarbazide Hydrochloride on the P-quinones 1 Contribution to the Study of Hydroxyazo-bodies. by James Alexander Russell Henderson,B.Sc.„ ProQuest Number: 13915827 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13915827 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Contents. Introduction and theory 3 Conclusions 17 Experimental 3.9 Action of ultra-violet light 50 Optical investigation 53. A Contribution to the Study of Hydroxyazo-bodies. The researches which have been made in recent years with regard to the constitution of "quinone hydrazones" prove conclusively that these bodies under the conditions of examination are not quinone derivatives but hydroxyazo-compounds; where quinone hydrazones might have been expected rearrangement invariably seems to take place with formation of hydroxyazo-bodies. Thus Borsche (Ann.1907, 357,171) found that by condensing o-nitrophenylhydrazine with p-benzoquinone there resulted not the quinone hydrazone but the isomeric hydroxyazo-body:- o h2h n h c 6h 4no2 n :n c 6h 4k o 2 o o 0 OH The spectroscopic evidence,at any rate for the para-quinones, also supports the view that where quinone hydrazones might be ex pected hydroxyazo-bodies are formed.