Barataria-Terrebonne Estuary Bird Curriculum
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Chromosome Painting in Three Species of Buteoninae: a Cytogenetic Signature Reinforces the Monophyly of South American Species
Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species Edivaldo Herculano C. de Oliveira1,2,3*, Marcella Mergulha˜o Tagliarini4, Michelly S. dos Santos5, Patricia C. M. O’Brien3, Malcolm A. Ferguson-Smith3 1 Laborato´rio de Cultura de Tecidos e Citogene´tica, SAMAM, Instituto Evandro Chagas, Ananindeua, PA, Brazil, 2 Faculdade de Cieˆncias Exatas e Naturais, ICEN, Universidade Federal do Para´, Bele´m, PA, Brazil, 3 Cambridge Resource Centre for Comparative Genomics, Cambridge, United Kingdom, 4 Programa de Po´s Graduac¸a˜oem Neurocieˆncias e Biologia Celular, ICB, Universidade Federal do Para´, Bele´m, PA, Brazil, 5 PIBIC – Universidade Federal do Para´, Bele´m, PA, Brazil Abstract Buteoninae (Falconiformes, Accipitridae) consist of the widely distributed genus Buteo, and several closely related species in a group called ‘‘sub-buteonine hawks’’, such as Buteogallus, Parabuteo, Asturina, Leucopternis and Busarellus, with unsolved phylogenetic relationships. Diploid number ranges between 2n = 66 and 2n = 68. Only one species, L. albicollis had its karyotype analyzed by molecular cytogenetics. The aim of this study was to present chromosomal analysis of three species of Buteoninae: Rupornis magnirostris, Asturina nitida and Buteogallus meridionallis using fluorescence in situ hybridization (FISH) experiments with telomeric and rDNA probes, as well as whole chromosome probes derived from Gallus gallus and Leucopternis albicollis. The three species analyzed herein showed similar karyotypes, with 2n = 68. Telomeric probes showed some interstitial telomeric sequences, which could be resulted by fusion processes occurred in the chromosomal evolution of the group, including the one found in the tassociation GGA1p/GGA6. -

Bird Species and Climate Change
Bird Species and Climate Change The Global Status Report: A synthesis of current scientific understanding of anthropogenic climate change impacts on global bird species now, and projected future effects. Report Climate Risk Climate A Climate Risk Pty Ltd are specialist advisors to business and government on risk, opportunity and adaptation to climate change. Climate Risk www.climaterisk.net Climate Risk Pty Limited (Australia) Level 1, 36 Lauderdale Avenue Fairlight, NSW 2094 Tel: + 61 2 8003 4514 Brisbane: + 61 7 3102 4513 www.climaterisk.net Climate Risk Europe Limited London: + 44 20 8144 4510 Manchester: + 44 16 1273 2474 This report was prepared by: Janice Wormworth BSc MA [email protected] Dr Karl Mallon BSc PhD Tel: + 61 412 257 521 [email protected] Bird Species and Climate Change: The Global Status Report version 1.0 A report to: World Wide Fund for Nature The authors of this report would like to thank our peer reviewers, including Dr. Lara Hansen and Prof. Rik Leemans. We would also like to thank Corin Millais, Paul Toni and Gareth Johnston for their input. ISBN: 0-646-46827-8 Designed by Digital Eskimo www.digitaleskimo.net Disclaimer While every effort has been made to ensure that this document and the sources of information used here are free of error, the authors: Are not responsible, or liable for, the accuracy, currency and reliability of any information provided in this publication; Make no express or implied representation of warranty that any estimate of forecast will be achieved or that any statement -

Impact of Climatic Change on Avian Populations: Implication for Long Term Conservation in Wildlife Genetic Resources
American Journal of Bioscience and Bioengineering 2017; 5(1): 23-33 http://www.sciencepublishinggroup.com/j/bio doi: 10.11648/j.bio.20170501.15 ISSN: 2328-5885 (Print); ISSN: 2328-5893 (Online) Review Article Impact of Climatic Change on Avian Populations: Implication for Long Term Conservation in Wildlife Genetic Resources Weldemariam Tesfahunegny1, *, Getachew Mulualem2 1Ethiopian Biodiversity Institute, Animal Biodiversity Directorate, Addis Ababa, Ethiopia 2Mekelle Biodiversity Centre, Animal Biodiversity, Wildlife Studies, Tigray, Ethiopia Email address: [email protected] (W. Tesfahunegny) *Corresponding author To cite this article: Weldemariam Tesfahunegny, Getachew Mulualem. Impact of Climatic Change on Avian Populations: Implication for Long Term Conservation in Wildlife Genetic Resources. American Journal of Bioscience and Bioengineering. Vol. 5, No. 1, 2017, pp. 23-33. doi: 10.11648/j.bio.20170501.15 Received: September 19, 2016; Accepted: January 4, 2017; Published: February 21, 2017 Abstract: Climate change is a multidimensional challenged on bird population indirectly. Wildlife species are not equally at risk when facing climate change. Several species-specific attributes have been identified as increasing species vulnerability to climate change, including diets, migratory strategy, main habitat types and ecological specialization. Conservationists mostly used small number of species as surrogates to help them tackle conservation problems. Having served as reliable indicators of environmental change for centuries, bird populations now indicate that global warming have set in motion as a powerful chain of effects in ecosystems worldwide. The effect of climate change on the variation of species distribution is a matter of conservation emphasis globally. Successful management and conservation of species and their respective habitat dilemma under climate change rely on our ability to model species-habitat interaction and predict species distribution under changing environmental condition. -
Avian Taxonomy
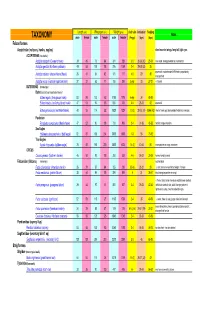
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64 -
Pd Films List 0824
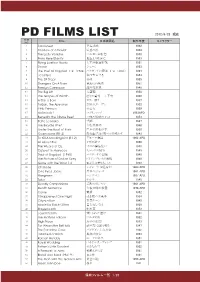
PD FILMS LIST 2012/8/23 現在 FILM Title 日本映画名 制作年度 キャラクター NO 1 Sabouteur 逃走迷路 1942 2 Shadow of a Doubt 疑惑の影 1943 3 The Lady Vanishe バルカン超特急 1938 4 From Here Etanity 地上より永遠に 1953 5 Flying Leather Necks 太平洋航空作戦 1951 6 Shane シェーン 1953 7 The Thief Of Bagdad 1・2 (1924) バクダッドの盗賊 1・2 (1924) 1924 8 I Confess 私は告白する 1953 9 The 39 Steps 39夜 1935 10 Strangers On A Train 見知らぬ乗客 1951 11 Foreign Correspon 海外特派員 1940 12 The Big Lift 大空輸 1950 13 The Grapes of Wirath 怒りの葡萄 上下有 1940 14 A Star Is Born スター誕生 1937 15 Tarzan, the Ape Man 類猿人ターザン 1932 16 Little Princess 小公女 1939 17 Mclintock! マクリントック 1963APD 18 Beneath the 12Mile Reef 12哩の暗礁の下に 1953 19 PePe Le Moko 望郷 1937 20 The Bicycle Thief 自転車泥棒 1948 21 Under The Roof of Paris 巴里の屋根の根 下 1930 22 Ossenssione (R1.2) 郵便配達は2度ベルを鳴らす 1943 23 To Kill A Mockingbird (R1.2) アラバマ物語 1962 APD 24 All About Eve イヴの総て 1950 25 The Wizard of Oz オズの魔法使い 1939 26 Outpost in Morocco モロッコの城塞 1949 27 Thief of Bagdad (1940) バクダッドの盗賊 1940 28 The Picture of Dorian Grey ドリアングレイの肖像 1949 29 Gone with the Wind 1.2 風と共に去りぬ 1.2 1939 30 Charade シャレード(2種有り) 1963 APD 31 One Eyed Jacks 片目のジャック 1961 APD 32 Hangmen ハングマン 1987 APD 33 Tulsa タルサ 1949 34 Deadly Companions 荒野のガンマン 1961 APD 35 Death Sentence 午後10時の殺意 1974 APD 36 Carrie 黄昏 1952 37 It Happened One Night 或る夜の出来事 1934 38 Cityzen Ken 市民ケーン 1945 39 Made for Each Other 貴方なしでは 1939 40 Stagecoach 駅馬車 1952 41 Jeux Interdits 禁じられた遊び 1941 42 The Maltese Falcon マルタの鷹 1952 43 High Noon 真昼の決闘 1943 44 For Whom the Bell tolls 誰が為に鐘は鳴る 1947 45 The Paradine Case パラダイン夫人の恋 1942 46 I Married a Witch 奥様は魔女 -

Investing in America's Natural Resources
Investing in America’s Natural Resources The Urgent Need for Climate Change Legislation May 2008 Prepared by: Douglas B. Inkley, Ph.D., Senior Scientist National Wildlife Federation 11100 Wildlife Center Drive ©2008 by the National Wildlife Federation. Reston, VA 20190 All rights reserved. 703-438-6000 www.nwf.org Larry J. Schweiger President and Chief Executive Officer National Wildlife Federation Acknowledgments On the National Wildlife Federation staff, I especially thank Jim Adams, Barbara Bramble, Juanita Constible, Ben Deeble, Sarah Gannon-Nagle, Patty Glick, Laura Hartt, Pat Lavin, Steve Malloch, David McArthur, Michael Murray, Amanda Staudt and Garrit Voggesser for contributing their ex- pertise and research on various ecosystems. The guidance and special skills of Laura Hickey, Jennifer Jones, John Kostyack, Aislinn Maestas and Karla Raettig were essential to successful completion of this report. Thank you to Joe Barbato of Barbato Associates for early drafts from which this report was developed and to Julie Young of Young Design for her design work. Thank you to all the professionals in resources conservation who are working so hard to conserve and protect the nation’s natural resources. FRONT COVER PHOTOGRAPHS: iStock Investing in America’s Natural Resources The Urgent Need for Climate Change Legislation AmeRIcA is blessed with an abundance of natural resources: from the fertile Great Plains to the stunning coasts teeming with fish, from the vast forests that span much of our country to the unparalleled freshwater reservoir of the Great Lakes. These natural resources are essential for our food, shelter and economic vitality, provide for our physical and spiritual well- being, and are integral to what it means to be American. -

SMCSP & SMCSN Wildlife List.Xlsx
Appendix C: Wildlife list for Six Mile Cypress Slough and Six Mile Cypress North Preserves Designated Status Scientific Name Common Name FWC FWS FNAI MAMMALS Family: Didelphidae (opossums) Didelphis virginiana Virginia opossum Family: Dasypodidae (armadillos) Dasypus novemcinctus nine-banded armadillo * Family: Sciuridae (squirrels and their allies) Sciurus carolinensis eastern gray squirrel Sciurus niger avicennia Big Cypress fox squirrel T G5T2/S2 Family: Muridae (mice and rats) Peromyscus gossypinus cotton mouse Oryzomys palustris marsh rice rat Sigmodon hispidus hispid cotton rat Family: Leporidae (rabbits and hares) Sylvilagus palustris marsh rabbit Sylvilagus floridanus eastern cottontail Family: Talpidae (moles) Scalopus aquaticus eastern mole Family: Felidae (cats) Puma concolor coryi Florida panther E E G5T1/S1 Lynx rufus bobcat Felis silvestris domestic cat * Family: Canidae (wolves and foxes) Urocyon cinereoargenteus common gray fox Family: Ursidae (bears) Ursus americanus floridanus Florida black bear T G5T2/S2 Family: Procyonidae (raccoons) Procyon lotor raccoon Family: Mephitidae (skunks) Spilogale putorius eastern spotted skunk Mephitis mephitis striped skunk Family: Mustelidae (weasels, otters and relatives) Lutra canadensis northern river otter Family: Suidae (old world swine) Sus scrofa feral hog * Family: Cervidae (deer) Odocoileus virginianus white-tailed deer BIRDS Family: Anatidae (swans, geese and ducks) Subfamily: Anatinae (dabbling ducks) Dendrocygna autumnalis black-bellied whistling duck Cairina moschata muscovy -

Climate Change and Birds: Perspectives and Prospects from Southern Africa
Ostrich 2004, 75(4): 295–308 Copyright © NISC Pty Ltd Printed in South Africa — All rights reserved OSTRICH ISSN 0030–6525 Please respond to queries in red — Thanks Climate change and birds: perspectives and prospects from southern Africa Robert E Simmons1*, Phoebe Barnard2, WRJ Dean1, Guy F Midgley3, Wilfried Thuiller3 and Greg Hughes3 1 Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7700, Cape Town, South Africa 2 Global Invasive Species Programme, National Botanical Institute, Private Bag X7, Claremont 7735, Cape Town, South Africa 3 Climate Change Research Group, Ecology and Conservation, Kirstenbosch Research Centre, South African National Biodiversity Institute, Private Bag X7, Claremont 7735, Cape Town, South Africa *Corresponding author, e-mail: [email protected] Global climate warming, now conclusively linked to anthropogenically-increased CO2 levels in the earth’s atmosphere, has already had impacts on the earth’s biodiversity and is predicted to threaten more than 1 million species with extinction by 2050. Climate change in southern Africa is expected to involve higher temperatures and lower rainfall, with less predictability and a greater frequency of severe storms, fires and El Niño events. The predicted changes to birds in Africa — the continent most at risk from climate change — have hardly been explored, yet birds and many other vertebrates face uncertain futures. Here, in one of the first focused analyses of the correlates of climate change vulnerability in southern African birds, we offer a wide- ranging perspective on which species may be most at risk, and explore which traits may influence the adaptability or extinc- tion risk of bird species. -

Increasing Temperature, Not Mean Temperature, Is a Cue for Avian Timing of Reproduction
vol. 179, no. 2 the american naturalist february 2012 E-Article Increasing Temperature, Not Mean Temperature, Is a Cue for Avian Timing of Reproduction Sonja V. Schaper,1,* Alistair Dawson,2 Peter J. Sharp,3 Phillip Gienapp,4 Samuel P. Caro,1 and Marcel E. Visser1 1. Department of Animal Ecology, Netherlands Institute of Ecology (NIOO-KNAW), P.O. Box 50, 6700 AB Wageningen, The Netherlands; 2. Centre for Ecology and Hydrology, Bush Estate, Penicuik, Midlothian EH26 0QB, United Kingdom; 3. Roslin Institute and Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh, Midlothian EH25 9RG, United Kingdom; 4. Ecological Genetics Research Unit, Department of Biosciences, University of Helsinki, Viikinkaari 11, FIN-00014 Helsinki, Finland Submitted March 1, 2011; Accepted September 22, 2011; Electronically published December 20, 2011 Online enhancements: appendixes. Dryad data: http://dx.doi.org/10.5061/dryad.22pm2619. nutrient availability (Lack 1968; Perrins 1970; Verhulst and abstract: Timing of reproduction in temperate-zone birds is Tinbergen 1991). In this period, birds have to lay eggs and strongly correlated with spring temperature, with an earlier onset of breeding in warmer years. Females adjust their timing of egg laying raise their offspring. After breeding, they require energy between years to be synchronized with local food sources and thereby for molt, which has to be completed before conditions optimize reproductive output. However, climate change currently deteriorate. The timing of these connected life-cycle stages disrupts the link between predictive environmental cues and spring is crucial to maximize individual fitness (Wingfield and phenology. To investigate direct effects of temperature on the decision Kenagy 1991; Grieco et al. -

Potential Impacts of Climate Change on Neotropical Migrants: Management Implications1
Potential Impacts of Climate Change on Neotropical Migrants: Management Implications1 Jeff T. Price2 and Terry L. Root3 ________________________________________ Abstract The world is warming. Over the last 100 years, the the century. Limited data from other sources indicates global average temperature has increased by approxi- that the global mean temperature for the 20th century is mately 0.7°C. The United Nations Intergovernmental at least as warm as any other period since at least 1400 Panel on Climate Change projects a further increase in AD (IPCC 2001). global mean temperatures of between 1.4° - 5.8° C by the year 2100. How will climate change affect Neo- Since pre-industrial times, there have been significant tropical migrants? Models of changes in the breeding increases in the amount of carbon dioxide (CO2), distributions of North American birds predict that most methane (CH4), and nitrous oxide (N2O) in the atmos- species will undergo some shift in their ranges. In parts phere, leading to an enhancement of the Earth’s natural of northern Minnesota and southern Ontario, this could greenhouse effect. These increases in greenhouse gases lead to an avifauna with as many as 16 fewer species of can largely be attributed to human activities, including wood warblers than currently occur. Unless all compo- burning of fossil fuels and land use changes (such as nents of the ecosystem change at the same rate, an deforestation). In 1996, the Intergovernmental Panel on unlikely prospect, this potential disruption of the eco- Climate Change published the statement that “the system could lead to major impacts on forest health. -

Repeated Sequence Homogenization Between the Control and Pseudo
Repeated Sequence Homogenization Between the Control and Pseudo-Control Regions in the Mitochondrial Genomes of the Subfamily Aquilinae LUIS CADAH´IA1*, WILHELM PINSKER1, JUAN JOSE´ NEGRO2, 3 4 1 MIHAELA PAVLICEV , VICENTE URIOS , AND ELISABETH HARING 1Molecular Systematics, 1st Zoological Department, Museum of Natural History Vienna, Vienna, Austria 2Department of Evolutionary Ecology, Estacio´n Biolo´gica de Don˜ana, Sevilla, Spain 3Centre of Ecological and Evolutionary Synthesis (CEES), Department of Biology, Faculty for Natural Sciences and Math, University of Oslo, Oslo, Norway 4Estacio´n Biolo´gica Terra Natura (Fundacio´n Terra Natura—CIBIO), Universidad de Alicante, Alicante, Spain ABSTRACT In birds, the noncoding control region (CR) and its flanking genes are the only parts of the mitochondrial (mt) genome that have been modified by intragenomic rearrangements. In raptors, two noncoding regions are present: the CR has shifted to a new position with respect to the ‘‘ancestral avian gene order,’’ whereas the pseudo-control region (CCR) is located at the original genomic position of the CR. As possible mechanisms for this rearrangement, duplication and transposition have been considered. During characterization of the mt gene order in Bonelli’s eagle Hieraaetus fasciatus, we detected intragenomic sequence similarity between the two regions supporting the duplication hypothesis. We performed intra- and intergenomic sequence comparisons in H. fasciatus and other falconiform species to trace the evolution of the noncoding mtDNA regions in Falconiformes. We identified sections displaying different levels of similarity between the CR and CCR. On the basis of phylogenetic analyses, we outline an evolutionary scenario of the underlying mutation events involving duplication and homogenization processes followed by sporadic deletions. -

Molecular Phylogenetics of the Buteonine Birds of Prey (Accipitridae)
'e Auk 304(2):304–315, 2008 )e American Ornithologists’ Union, 2008. Printed in USA. MOLECULAR PHYLOGENETICS OF THE BUTEONINE BIRDS OF PREY (ACCIPITRIDAE) HEATHER R. L. LERNER,1 MATTHEW C. KLAVER, AND DAVID P. MINDELL2 Museum of Zoology and Department of Ecology and Evolutionary Biology, University of Michigan, 1109 Geddes Avenue, Ann Arbor, Michigan 48109, USA A.—Phylogenetic relationships among birds of prey in thhee subbffamily Buteoninae are not fully established but are of par- ticular interest because the Buteoninae constitute one of the largest accipitrid subgroups and include multiple species of conservation concern. Genera previously included within the Buteoninae are Buteo, Leucopternis, Buteogallus, Harpyhaliaetus, Busarellus, Parabu- teo, Geranoaetus, Geranospiza, Ictinia, Rostrhamus, Kaupifalco, and Butastur. We analyzed representatives from all buteonine genera and most non-Buteo (i.e., “sub-buteo”) species with , bases of nuclear and mitochondrial DNA and found non-monophyly for the nominal genera Buteo, Buteogallus, and Leucopternis. )e Old World Lizard Buzzard (Kaupifalco monogrammicus) is not closely re- lated to buteonine taxa but is sister to goshawks in the genera Melierax, Micronisus, and Urotriorchis. Another Old World genus, Butas- tur, is sister to the clade including all other buteonine genera mentioned above. Investigation of several “superspecies” complexes within the genus Leucopternis revealed non-monophyly for the four subspecies of White Hawk (L. albicollis). On the basis of mitochondrial data, L. a. albicollis forms a clade with L. polionotus, whereas L. a. costaricensis, L. a. ghiesbreghti, and L. a. williaminae form a clade with L. occidentalis. Among taxa included as outgroups, we found two species in the genus Circus to be clearly nested within a clade of Accipiter spp.