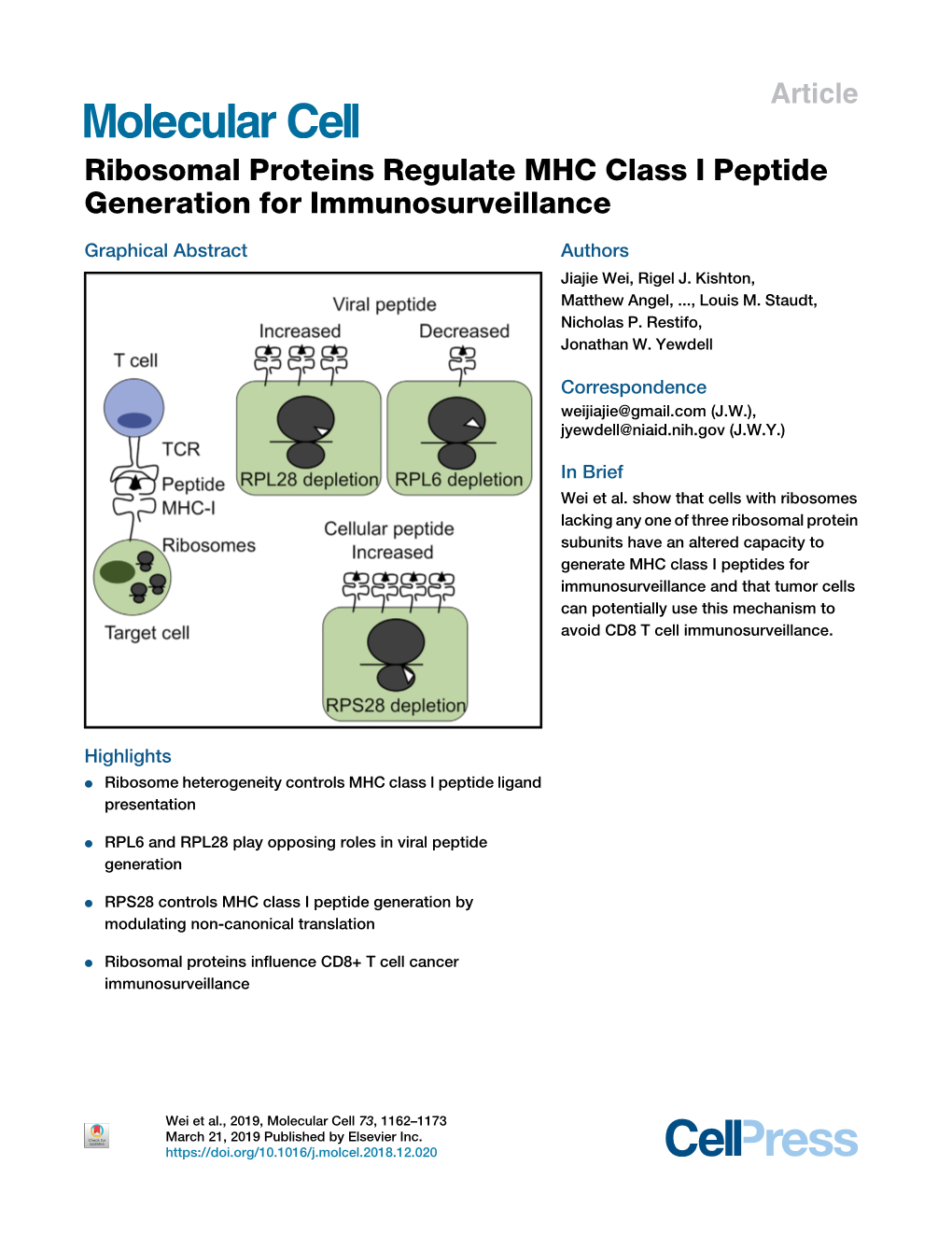
Ribosomal Proteins Regulate MHC Class I Peptide Generation for Immunosurveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allele-Specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish
Allele-specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish by Ailu Chen A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 1, 2015 Keywords: catfish, interspecific hybrids, allele-specific expression, ribosomal protein Copyright 2015 by Ailu Chen Approved by Zhanjiang Liu, Chair, Professor, School of Fisheries, Aquaculture and Aquatic Sciences Nannan Liu, Professor, Entomology and Plant Pathology Eric Peatman, Associate Professor, School of Fisheries, Aquaculture and Aquatic Sciences Aaron M. Rashotte, Associate Professor, Biological Sciences Abstract Interspecific hybridization results in a vast reservoir of allelic variations, which may potentially contribute to phenotypical enhancement in the hybrids. Whether the allelic variations are related to the downstream phenotypic differences of interspecific hybrid is still an open question. The recently developed genome-wide allele-specific approaches that harness high- throughput sequencing technology allow direct quantification of allelic variations and gene expression patterns. In this work, I investigated allele-specific expression (ASE) pattern using RNA-Seq datasets generated from interspecific catfish hybrids. The objective of the study is to determine the ASE genes and pathways in which they are involved. Specifically, my study investigated ASE-SNPs, ASE-genes, parent-of-origins of ASE allele and how ASE would possibly contribute to heterosis. My data showed that ASE was operating in the interspecific catfish system. Of the 66,251 and 177,841 SNPs identified from the datasets of the liver and gill, 5,420 (8.2%) and 13,390 (7.5%) SNPs were identified as significant ASE-SNPs, respectively. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Nucleolin and Its Role in Ribosomal Biogenesis
NUCLEOLIN: A NUCLEOLAR RNA-BINDING PROTEIN INVOLVED IN RIBOSOME BIOGENESIS Inaugural-Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf vorgelegt von Julia Fremerey aus Hamburg Düsseldorf, April 2016 2 Gedruckt mit der Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf Referent: Prof. Dr. A. Borkhardt Korreferent: Prof. Dr. H. Schwender Tag der mündlichen Prüfung: 20.07.2016 3 Die vorgelegte Arbeit wurde von Juli 2012 bis März 2016 in der Klinik für Kinder- Onkologie, -Hämatologie und Klinische Immunologie des Universitätsklinikums Düsseldorf unter Anleitung von Prof. Dr. A. Borkhardt und in Kooperation mit dem ‚Laboratory of RNA Molecular Biology‘ an der Rockefeller Universität unter Anleitung von Prof. Dr. T. Tuschl angefertigt. 4 Dedicated to my family TABLE OF CONTENTS 5 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................... 5 LIST OF FIGURES ......................................................................................................10 LIST OF TABLES .......................................................................................................12 ABBREVIATION .........................................................................................................13 ABSTRACT ................................................................................................................19 ZUSAMMENFASSUNG -

Comprehensive Array CGH of Normal Karyotype Myelodysplastic
Leukemia (2011) 25, 387–399 & 2011 Macmillan Publishers Limited All rights reserved 0887-6924/11 www.nature.com/leu LEADING ARTICLE Comprehensive array CGH of normal karyotype myelodysplastic syndromes reveals hidden recurrent and individual genomic copy number alterations with prognostic relevance A Thiel1, M Beier1, D Ingenhag1, K Servan1, M Hein1, V Moeller1, B Betz1, B Hildebrandt1, C Evers1,3, U Germing2 and B Royer-Pokora1 1Institute of Human Genetics and Anthropology, Medical Faculty, Heinrich Heine University, Duesseldorf, Germany and 2Department of Hematology, Oncology and Clinical Immunology, Heinrich Heine University, Duesseldorf, Germany About 40% of patients with myelodysplastic syndromes (MDSs) 40–50% of MDS cases have a normal karyotype. MDS patients present with a normal karyotype, and they are facing different with a normal karyotype and low-risk clinical parameters are courses of disease. To advance the biological understanding often assigned into the IPSS low and intermediate-1 risk groups. and to find molecular prognostic markers, we performed a high- resolution oligonucleotide array study of 107 MDS patients In the absence of genetic or biological markers, prognostic (French American British) with a normal karyotype and clinical stratification of these patients is difficult. To better prognosticate follow-up through the Duesseldorf MDS registry. Recurrent these patients, new parameters to identify patients at higher risk hidden deletions overlapping with known cytogenetic aberra- are urgently needed. With the more recently introduced modern tions or sites of known tumor-associated genes were identi- technologies of whole-genome-wide surveys of genetic aberra- fied in 4q24 (TET2, 2x), 5q31.2 (2x), 7q22.1 (3x) and 21q22.12 tions, it is hoped that more insights into the biology of disease (RUNX1, 2x). -

Supplementary Table
Supporting information Additional Supporting Information may be found in the online version of this article: Supplementary Table S1: List of deregulated genes in serum of cancer patients in comparision to serum of healthy individuals (p < 0.05, logFC ≥ 1). ENTREZ Gene ID Symbol Gene Name logFC p-value q-value 8407 TAGLN2 transgelin 2 3,78 3,40E-07 0,0022 7035 TFPI tissue factor pathway inhibitor (lipoprotein-associated coagulation inhibitor) 3,53 4,30E-05 0,022 28996 HIPK2 homeodomain interacting protein kinase 2 3,49 2,50E-06 0,0066 3690 ITGB3 integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) 3,48 0,00053 0,081 7035 TFPI tissue factor pathway inhibitor (lipoprotein-associated coagulation inhibitor) 3,45 1,80E-05 0,014 4900 NRGN neurogranin (protein kinase C substrate, RC3) 3,32 0,00012 0,037 10398 MYL9 myosin, light chain 9, regulatory 3,22 8,20E-06 0,011 3796 KIF2A kinesin heavy chain member 2A 3,14 0,00015 0,04 5476 CTSA cathepsin A 3,08 0,00015 0,04 6648 SOD2 superoxide dismutase 2, mitochondrial 3,07 4,20E-06 0,0077 2982 GUCY1A3 guanylate cyclase 1, soluble, alpha 3 3,07 0,0015 0,13 8459 TPST2 tyrosylprotein sulfotransferase 2 3,05 0,00043 0,074 2983 GUCY1B3 guanylate cyclase 1, soluble, beta 3 3,04 3,70E-05 0,021 145781 GCOM1 GRINL1A complex locus 3,02 0,00027 0,059 10611 PDLIM5 PDZ and LIM domain 5 2,87 1,80E-05 0,014 5567 PRKACB protein kinase, cAMP-dependent, catalytic, beta 2,85 0,0015 0,13 25907 TMEM158 transmembrane protein 158 (gene/pseudogene) 2,84 0,0068 0,27 8848 TSC22D1 TSC22 domain family, member 1 2,83 0,00058 0,084 26 351 APP amyloid beta (A4) precursor protein 2,82 0,00018 0,045 9240 PNMA1 paraneoplastic antigen MA1 2,78 0,00028 0,06 400073 C12orf76 chromosome 12 open reading frame 76 2,78 0,00069 0,091 649260 ILMN_35781 PREDICTED: Homo sapiens similar to LIM and senescent cell antigen-like domains 1 (LOC649260), mRNA. -

Human C7orf50 ORF Mammalian Expression Plasmid, C-Gfpspark Tag
Human C7orf50 ORF mammalian expression plasmid, C-GFPSpark tag Catalog Number: HG14587-ACG General Information Plasmid Resuspension protocol Gene : chromosome 7 open reading frame 50 1. Centrifuge at 5,000×g for 5 min. Official Symbol : C7ORF50 2. Carefully open the tube and add 100 l of sterile water to Synonym : YCR016W dissolve the DNA. Source : Human 3. Close the tube and incubate for 10 minutes at room cDNA Size: 585bp temperature. RefSeq : BC006224 4. Briefly vortex the tube and then do a quick spin to Description concentrate the liquid at the bottom. Speed is less than Lot : Please refer to the label on the tube 5000×g. Vector : pCMV3-C-GFPSpark 5. Store the plasmid at -20 ℃. Shipping carrier : Each tube contains approximately 10 μg of lyophilized plasmid. The plasmid is ready for: Storage : • Restriction enzyme digestion The lyophilized plasmid can be stored at ambient temperature for three months. • PCR amplification Quality control : • E. coli transformation The plasmid is confirmed by full-length sequencing with primers • DNA sequencing in the sequencing primer list. Sequencing primer list : E.coli strains for transformation (recommended but not limited) pCMV3-F: 5’ CAGGTGTCCACTCCCAGGTCCAAG 3’ Most commercially available competent cells are appropriate for pcDNA3-R : 5’ GGCAACTAGAAGGCACAGTCGAGG 3’ the plasmid, e.g. TOP10, DH5α and TOP10F´. Or Forward T7 : 5’ TAATACGACTCACTATAGGG 3’ ReverseBGH : 5’ TAGAAGGCACAGTCGAGG 3’ pCMV3-F and pcDNA3-R are designed by Sino Biological Inc. Customers can order the primer pair from any oligonucleotide supplier. Manufactured By Sino Biological Inc., FOR RESEARCH USE ONLY. NOT FOR USE IN HUMANS. Fax :+86-10-51029969 Tel:+86- 400-890-9989 http://www.sinobiological.com Human C7orf50 ORF mammalian expression plasmid, C-GFPSpark tag Catalog Number: HG14587-ACG Vector Information All of the pCMV vectors are designed for high-level stable and transient expression in mammalian hosts. -

High Constitutive Cytokine Release by Primary Human Acute Myeloid Leukemia Cells Is Associated with a Specific Intercellular Communication Phenotype
Supplementary Information High Constitutive Cytokine Release by Primary Human Acute Myeloid Leukemia Cells Is Associated with a Specific Intercellular Communication Phenotype Håkon Reikvam 1,2,*, Elise Aasebø 1, Annette K. Brenner 2, Sushma Bartaula-Brevik 1, Ida Sofie Grønningsæter 2, Rakel Brendsdal Forthun 2, Randi Hovland 3,4 and Øystein Bruserud 1,2 1 Department of Clinical Science, University of Bergen, 5020, Bergen, Norway 2 Department of Medicine, Haukeland University Hospital, 5021, Bergen, Norway 3 Department of Medical Genetics, Haukeland University Hospital, 5021, Bergen, Norway 4 Institute of Biomedicine, University of Bergen, 5020, Bergen, Norway * Correspondence: [email protected]; Tel.: +55-97-50-00 J. Clin. Med. 2019, 8, x 2 of 36 Figure S1. Mutational studies in a cohort of 71 AML patients. The figure shows the number of patients with the various mutations (upper), the number of mutations in for each patient (middle) and the number of main classes with mutation(s) in each patient (lower). 2 J. Clin. Med. 2019, 8, x; doi: www.mdpi.com/journal/jcm J. Clin. Med. 2019, 8, x 3 of 36 Figure S2. The immunophenotype of primary human AML cells derived from 62 unselected patients. The expression of the eight differentiation markers CD13, CD14, CD15, CD33, CD34, CD45, CD117 and HLA-DR was investigated for 62 of the 71 patients included in our present study. We performed an unsupervised hierarchical cluster analysis and identified four patient main clusters/patient subsets. The mutational profile for each f the 62 patients is also given (middle), no individual mutation of main class of mutations showed any significant association with any of the for differentiation marker clusters (middle). -

NF-Y Controls Fidelity of Transcription Initiation at Gene Promoters
ARTICLE https://doi.org/10.1038/s41467-019-10905-7 OPEN NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region Andrew J. Oldfield 1,6, Telmo Henriques1,2,8, Dhirendra Kumar1,8, Adam B. Burkholder3,8, Senthilkumar Cinghu1, Damien Paulet4,5, Brian D. Bennett3, Pengyi Yang 1,7, Benjamin S. Scruggs1, Christopher A. Lavender3, Eric Rivals 4,5, Karen Adelman1,2 & Raja Jothi1 1234567890():,; Faithful transcription initiation is critical for accurate gene expression, yet the mechanisms underlying specific transcription start site (TSS) selection in mammals remain unclear. Here, we show that the histone-fold domain protein NF-Y, a ubiquitously expressed transcription factor, controls the fidelity of transcription initiation at gene promoters in mouse embryonic stem cells. We report that NF-Y maintains the region upstream of TSSs in a nucleosome- depleted state while simultaneously protecting this accessible region against aberrant and/or ectopic transcription initiation. We find that loss of NF-Y binding in mammalian cells disrupts the promoter chromatin landscape, leading to nucleosomal encroachment over the canonical TSS. Importantly, this chromatin rearrangement is accompanied by upstream relocation of the transcription pre-initiation complex and ectopic transcription initiation. Further, this phenomenon generates aberrant extended transcripts that undergo translation, disrupting gene expression profiles. These results suggest NF-Y is a central player in TSS selection in metazoans and highlight the deleterious consequences of inaccurate transcription initiation. 1 Epigenetics and Stem Cell Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, Durham, NC 27709, USA. 2 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA. -

The Transcriptional and Epigenetic Role of Brd4 in the Regulation of the Cellular Stress Response
THE TRANSCRIPTIONAL AND EPIGENETIC ROLE OF BRD4 IN THE REGULATION OF THE CELLULAR STRESS RESPONSE INAUGURAL-DISSERTATION to obtain the academic degree Doctor rerum naturalium (Dr. rer. nat.) submitted to the Department of Biology, Chemistry and Pharmacy of Freie Universität Berlin by Michelle Hussong from Zweibrücken 2015 Die vorliegende Arbeit wurde im Zeitraum von Juli 2012 bis September 2015 am Max- Planck-Institut für Molekulare Genetik in Berlin sowie an der Universität zu Köln unter der Leitung von Frau Prof. Dr. Dr. Michal-Ruth Schweiger angefertigt. 1. Gutachter: Prof. Dr. Dr. Michal-Ruth Schweiger 2. Gutachter: Prof. Dr. Rupert Mutzel Disputation am 07.12.2015 ACKNOWLEDGMENT ACKNOWLEDGMENT This dissertation would not have been possible without the guidance and the help of many people who in one way or another contributed to the preparation and completion of this study. Firstly, I would like to express my sincere gratitude to my advisor Prof. Dr. Dr. Michal-Ruth Schweiger, for her continuous support throughout my PhD study, for her patience, motivation, and immense knowledge. I am eminently thankful for the multiple possibilities she gave me to work on this interesting and challenging field of research. I also want to thank Professor Dr. Rupert Mutzel for taking the time of being my second supervisor. My sincere thanks also goes to Prof. Dr. Hans Lehrach for having given me the opportunity to do my PhD thesis in the extraordinary and inspiring environment at the Max-Planck- Institute for Molecular Genetics in Berlin. Especially, the multitude of technologies and knowledge in his department made my work successful. -

RNA–Protein Interaction Mapping Via MS2- Or Cas13-Based APEX Targeting
RNA–protein interaction mapping via MS2- or Cas13-based APEX targeting Shuo Hana,b,c,1, Boxuan Simen Zhaoa,b,c,1, Samuel A. Myersd, Steven A. Carrd, Chuan Hee,f, and Alice Y. Tinga,b,c,2 aDepartment of Genetics, Chan Zuckerberg Biohub, Stanford University, Stanford, CA 94305; bDepartment of Biology, Chan Zuckerberg Biohub, Stanford University, Stanford, CA 94305; cDepartment of Chemistry, Chan Zuckerberg Biohub, Stanford University, Stanford, CA 94305; dThe Broad Institute of Massachusetts Institute of Technology and Harvard University, Cambridge, MA 02142; eDepartment of Chemistry, Institute for Biophysical Dynamics, Howard Hughes Medical Institute, University of Chicago, Chicago, IL 60637; and fDepartment of Biochemistry and Molecular Biology, Institute for Biophysical Dynamics, Howard Hughes Medical Institute, University of Chicago, Chicago, IL 60637 Edited by Robert H. Singer, Albert Einstein College of Medicine, Bronx, NY, and approved July 24, 2020 (received for review April 8, 2020) RNA–protein interactions underlie a wide range of cellular pro- and oncogenesis by serving as the template for reverse transcrip- cesses. Improved methods are needed to systematically map RNA– tion of telomeres (26, 27). While hTR’s interaction with the protein interactions in living cells in an unbiased manner. We used telomerase complex has been extensively characterized (28), hTR two approaches to target the engineered peroxidase APEX2 to is present in stoichiometric excess over telomerase in cancer cells specific cellular RNAs for RNA-centered proximity biotinylation of (29) and is broadly expressed in tissues lacking telomerase protein protein interaction partners. Both an MS2-MCP system and an (30). These observations suggest that hTR could also function engineered CRISPR-Cas13 system were used to deliver APEX2 to outside of the telomerase complex (31), and uncharacterized the human telomerase RNA hTR with high specificity. -

High-Resolution Human Core-Promoter Prediction with Coreboost HM
Downloaded from genome.cshlp.org on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Methods High-resolution human core-promoter prediction with CoreBoost_HM Xiaowo Wang,1,2,3 Zhenyu Xuan,2,3 Xiaoyue Zhao,2 Yanda Li,1 and Michael Q. Zhang2,4 1MOE Key Laboratory of Bioinformatics and Bioinformatics Division, TNLIST/Department of Automation, Tsinghua University, Beijing 100084, China; 2Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA Correctly locating the gene transcription start site and the core-promoter is important for understanding transcriptional regulation mechanism. Here we have integrated specific genome-wide histone modification and DNA sequence features together to predict RNA polymerase II core-promoters in the human genome. Our new predictor CoreBoost_HM out- performs existing promoter prediction algorithms by providing significantly higher sensitivity and specificity at high resolution. We demonstrated that even though the histone modification data used in this study are from a specific cell type (CD4+ T-cell), our method can be used to identify both active and repressed promoters. We have applied it to search the upstream regions of microRNA genes, and show that CoreBoost_HM can accurately identify the known promoters of the intergenic microRNAs. We also identified a few intronic microRNAs that may have their own promoters. This result suggests that our new method can help to identify and characterize the core-promoters of both coding and noncoding genes. [Supplemental material is available online at www.genome.org.] Transcription initiation is a key step in the regulation of gene 2004, 2006; Zhang 2007). However, it is still a challenging prob- expression. -

Identification of MAZ As a Novel Transcription Factor Regulating Erythropoiesis
Identification of MAZ as a novel transcription factor regulating erythropoiesis Darya Deen1, Falk Butter2, Michelle L. Holland3, Vasiliki Samara4, Jacqueline A. Sloane-Stanley4, Helena Ayyub4, Matthias Mann5, David Garrick4,6,7 and Douglas Vernimmen1,7 1 The Roslin Institute and Royal (Dick) School of Veterinary Studies, University of Edinburgh, Easter Bush, Midlothian EH25 9RG, United Kingdom. 2 Institute of Molecular Biology (IMB), 55128 Mainz, Germany 3 Department of Medical and Molecular Genetics, School of Basic and Medical Biosciences, King's College London, London SE1 9RT, UK 4 MRC Molecular Haematology Unit, Weatherall Institute for Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DS, United Kingdom. 5 Department of Proteomics and Signal Transduction, Max Planck Institute of Biochemistry, 82152 Martinsried, Germany. 6 Current address : INSERM UMRS-976, Institut de Recherche Saint Louis, Université de Paris, 75010 Paris, France. 7 These authors contributed equally Correspondence: [email protected], [email protected] ADDITIONAL MATERIAL Suppl Fig 1. Erythroid-specific hypersensitivity regions DNAse-Seq. (A) Structure of the α-globin locus. Chromosomal position (hg38 genome build) is shown above the genes. The locus consists of the embryonic ζ gene (HBZ), the duplicated foetal/adult α genes (HBA2 and HBA1) together with two flanking pseudogenes (HBM and HBQ1). The upstream, widely-expressed gene, NPRL3 is transcribed from the opposite strand to that of the HBA genes and contains the remote regulatory elements of the α-globin locus (MCS -R1 to -R3) (indicated by red dots). Below is shown the ATAC-seq signal in human erythroblasts (taken from 8) indicating open chromatin structure at promoter and distal regulatory elements (shaded regions).