Immobilization of Lead and Zinc Leached from Mining
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Kabwe, Zambia
Mortimer Hays-Brandeis Traveling Fellowship Final Report, Hrvoje Slovene House Secrets: Industrial Tales in one of the World's Most Contaminated Cities: Kabwe, Zambia For the past five years, my primary focus in photography has been to document abandoned, nineteenth-century factories and industrial spaces, as well as to show the irreparable damage modem industrialism has had on the global environment and its population. By photographing the devastating effects on local neighborhoods wrought by factories and industry, my intention has been to show how seemingly isolated problems have contributed to the global environmental crisis we are witnessing today. To date this project has taken me to Manchester, England-the birthplace of the industrial revolution; to Cleveland, Ohio; and Zagreb, Croatia. With the generous help of the Mortimer Hays- Brandeis Traveling Fellowship, I expanded this project to include Kabwe, Zambia. The mining of lead there by a Chinese-based company continues to put the population of hundreds of thousands at serious risk. Through my photographs, I showed the impact of modem industrialism on the real, day-to-day experiences of people and their industrialized habitats. Although my original intention was to visit and document the effects oflead pollution in Kabwe, Zambia; Linfen, China; and Dzerzhinsk, Russia, I found a plethora of material in Zambia, and spent the majority of my time there. I first visited Kabwe in June 2007 and stayed there for three months. During that time I got in touch with local NGOs and familiarized myself with their work on the field. During that time I also became aware of the scale of the lead pollution problem on the location. -

A History of Mining in Broken Hill (Kabwe): 1902-1929 Buzandi Mufinda
A HISTORY OF MINING IN BROKEN HILL (KABWE): 1902-1929 BY BUZANDI MUFINDA THIS THESIS HAS BEEN SUBMITTED IN ACCORDANCE WITH THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS IN THE FACULTY OF THE HUMANITIES, FOR THE CENTRE FOR AFRICA STUDIES AT THE UNIVERSITY OF THE FREE STATE. FEBRUARY 2015 SUPERVISOR: PROF. I.R. PHIMISTER CO-SUPERVISOR: DR L. KOORTS DECLARATION I declare that the dissertation hereby submitted by me for the Master of Arts degree at the University of the Free State is my own independent work and has not previously been submitted by me at another university/faculty. I furthermore cede copyright of the dissertation in favour of the University of the Free State. Buzandi Mufinda i DEDICATION I dedicate this work to the memory of my late parents, Edward Mufinda, and Rosemary Mufinda, and to my niece Chipego Munene and hope one day she might follow in the footsteps of academia. ii ACKNOWLEDGEMENTS Glory is to the enabling power of the Almighty God whose hand has worked through many ways to make it possible for me to accomplish this study. I would like to express my sincere appreciation to my supervisor, Professor Ian Phimister, for the patient guidance, encouragement and advice he has provided throughout my time as his student. I have been extremely lucky to have a supervisor who cared so much about my work, and who responded to my questions and queries so promptly. To Doctor Lindie Koorts, your expertise in structuring and editing of this work continually amazed me. Thank you also for your moral support. -

Lead Intoxicated Children in Kabwe, Zambia
Lead intoxicated children in Kabwe, Zambia Stephan Bose-O’Reilly a,b*, John Yabe c, Joseph Makumba d, Paul Schutzmeier a, Bret Ericsone, Jack Caravanos e,f a. Institute and Policlinic of Occupational, Social and Environmental Medicine, WHO Collaborating Centre for Occupational Health, University Hospital, LMU Munich, Ziemssenstr. 1, D-80336 Munich, Germany ([email protected] muenchen.de) b. Department of Public Health, Health Services Research and Health Technology Assessment, UMIT – University for Health Sciences, Medical Informatics and Technology, Hall i.T., Austria ([email protected]) c. University of Zambia, School of Veterinary Medicine, Lusaka, Zambia ([email protected]) d. Misenge Environmental and Technical Services Ltd., ZCCM Investment Holdings Plc (ZCCM-IH), Kitwe, Zambia ([email protected]) e. Pure Earth, New York, USA ([email protected]) f. New York University of New York School of Public Health, New York, USA ([email protected]) Reports from the field 1 *Correspondence to: Stephan Bose-O’Reilly, Global Environmental Health, Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, WHO Collaborating Centre for Occupational Health, University Hospital, LMU Munich, Ziemssenstr. 1, D-80336 Munich, Germany, [email protected] Fon: ++49-89-44005 7687, Fax ++49-89-44005 4444 2 Abstract Kabwe is a lead contaminated mining town in Zambia. Kabwe has extensive lead contaminated soil and children in Kabwe ingest and inhale high quantities of this toxic dust. The aim of this paper is to analyze the health impact of this exposure for children. Health data from three existing studies were re-analyzed. -

Zambia Country Profile Monitoring, Reporting and Verification for REDD+
OCCASIONAL PAPER Zambia country profile Monitoring, reporting and verification for REDD+ Michael Day Davison Gumbo Kaala B. Moombe Arief Wijaya Terry Sunderland OCCASIONAL PAPER 113 Zambia country profile Monitoring, reporting and verification for REDD+ Michael Day Center for International Forestry Research Davison Gumbo Center for International Forestry Research Kaala B. Moombe Center for International Forestry Research Arief Wijaya Center for International Forestry Research Terry Sunderland Center for International Forestry Research Center for International Forestry Research (CIFOR) Occasional Paper 113 © 2014 Center for International Forestry Research Content in this publication is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License http://creativecommons.org/licenses/by-nc-nd/3.0/ ISBN 978-602-1504-42-0 Day M, Gumbo D, Moombe KB, Wijaya A and Sunderland T. 2014. Zambia country profile: Monitoring, reporting and verification for REDD+. Occasional Paper 113. Bogor, Indonesia: CIFOR. Photo by Terry Sunderland CIFOR Jl. CIFOR, Situ Gede Bogor Barat 16115 Indonesia T +62 (251) 8622-622 F +62 (251) 8622-100 E [email protected] cifor.org We would like to thank all donors who supported this research through their contributions to the CGIAR Fund. For a list of Fund donors please see: https://www.cgiarfund.org/FundDonors Any views expressed in this publication are those of the authors. They do not necessarily represent the views of CIFOR, the editors, the authors’ institutions, the financial sponsors or the -

Remediation and Improvement Project (P154683)
The World Bank Implementation Status & Results Report Zambia - Mining and Environmental Remediation and Improvement Project (P154683) Zambia - Mining and Environmental Remediation and Improvement Project (P154683) AFRICA EAST | Zambia | Environment, Natural Resources & the Blue Economy Global Practice | IBRD/IDA | Investment Project Financing | FY 2017 | Seq No: 9 | ARCHIVED on 29-Jun-2021 | ISR47258 | Public Disclosure Authorized Implementing Agencies: Ministry of Mines and Mineral Development, Ministry of Finance, Republic of Zambia Key Dates Key Project Dates Bank Approval Date: 16-Dec-2016 Effectiveness Date: 30-Nov-2017 Planned Mid Term Review Date: -- Actual Mid-Term Review Date: Original Closing Date: 30-Jun-2022 Revised Closing Date: 30-Jun-2022 pdoTable Project Development Objectives Public Disclosure Authorized Project Development Objective (from Project Appraisal Document) To reduce environmental health risks to the local population in critically polluted mining areas in Chingola, Kabwe, Kitwe and Mufulira municipalities, including lead exposure in Kabwe municipality Has the Project Development Objective been changed since Board Approval of the Project Objective? No Components Table Name Remediation of Contaminated Hotspots and Improvement of Environmental Infrastructure:(Cost $29.60 M) Enhancing Institutional capacity to strengthen environmental governance and compliance:(Cost $13.50 M) Reducing environmental health risks through localized interventions:(Cost $18.50 M) Public Disclosure Authorized Project Management, Monitoring and Evaluation:(Cost $4.00 M) Overall Ratings Name Previous Rating Current Rating Progress towards achievement of PDO Moderately Satisfactory Moderately Satisfactory Overall Implementation Progress (IP) Moderately Unsatisfactory Moderately Satisfactory Overall Risk Rating Substantial Substantial Implementation Status and Key Decisions The project has made good progress since the Midterm Review meeting which took place in Feb-March 2021. -

National Transportation System in the Republic of Zambia
World Maritime University The Maritime Commons: Digital Repository of the World Maritime University World Maritime University Dissertations Dissertations 1990 National transportation system in the Republic of Zambia Febby Mtonga WMU Follow this and additional works at: https://commons.wmu.se/all_dissertations Recommended Citation Mtonga, Febby, "National transportation system in the Republic of Zambia" (1990). World Maritime University Dissertations. 877. https://commons.wmu.se/all_dissertations/877 This Dissertation is brought to you courtesy of Maritime Commons. Open Access items may be downloaded for non- commercial, fair use academic purposes. No items may be hosted on another server or web site without express written permission from the World Maritime University. For more information, please contact [email protected]. WMU LIBRARY WORLD MARITIME UNIVERSITY Malmo ~ Sweden THE NATIONAL TRANSPORTATION SYSTEM IN THE REPUBLIC OF ZAMBIA by Febby Mtonga Zambia A paper submitted to the faculty of the World Maritime University in partial fulfillment of the requirements for the award of a MASTER OF SCIENCE DEGREE in GENERAL MARITIME ADMINISTRATION The views and contents expressed in this paper reflect entirely those of my own and are not to be construed as necessarily endorsed by the University Signed: Date : 0 5 I 11 j S O Assessed by: Professor J. Mlynarcz] World Maritime University Ilf Co-assessed by: U. 2).i TABLE OF CONTENTS 1 PREFACE i ACKNOWLEDGEMENT ii ABBREVIATIONS ... LIST OF MAPS AND APPENDICES iv CHAPTER 1 M • O • o Profile of the Republic of Zambia 1 1.1.0 Geographical Location of Zambia 1.2.0 Population 1.3.0 The Economy 1.3.1 Mining 1.3.2 Agriculture 3 1.3.3 Manufacturing 4 1.3.4 Transportation 7 1. -

Status, Priorities and Needs for T I Bl Il T I Sustainable Soil Management In
Status, priorities and needs for sustitaina ble so il managemen tit in Zambia SSStalin Sichinga Zamb ia Ag ricu ltu re Resea r ch Institute Introduction Zambia has an area of 750,000 km2 with about 13.9 million people and ample land resources 0ut of 9 million ha cultivable land, only 14% is cropped in any year About 55 - 60% of the land area is covered by natural forest and 6% of Zambia‘s land surface is covered by water. Agro-ecological regions and soil distribution The country is classified into three agro-ecological regions based on soil types, rainfall, and other climatic conditions Agro-Ecological Regions N Chiengi Kaputa Mpulungu W E Nchelenge Mbala Nakonde Mporokoso S Kawambwa Mungwi Isoka Scale 1: 2,500,000 Mwense Luwingu Kasama Chinsali Chilubi Mansa Chama LEGEND Samfya Milenge Mpika Regions Mwinilunga Chililabombwe Solwezi Agro-ecological Region I Chingola Mufulira Lundazi I Ka lul u shi Kitwe Ndola IIa Lufwanyama Luans hya Chavuma Serenje Mambwe Kabompo Masaiti IIb Mpongwe Zambezi Mufumbwe Chipata Kasempa Petauke Katete Chadiza III Annual rainfall is <750mm Kapiri Mposhi Mkushi Nyimba Kabwe Lukulu Kaoma Mumbwa Chibombo Kalabo Mongu Chongwe Lusaka Urban Luangwa Itezhi-Tezhi Kafue Namwala Mazabuka Senanga Monze KEY Siavonga Sesheke Gwembe Shangombo Choma District boundary e Kazungula Kalomo w g n o z a in Livingstone S 200 0 200 400 Kilometers December 2002 The region contains a diversity of soil types ranging from slightly acidic Nitosols to alkaline Luvisols with pockets of Vertisols, Arenosols, Leptosols and, Solonetz. The physical limitations of region I soils Hazards to erosion, lim ite d so il dept h in t he hills an d escarpment zones, presence of hardpans in the pan dambo areas, ppyoor workability in the cracking gy, clay soils, problems of crusting in most parts of the Southern province, low water-holding capacities and the problem of wetness in the valley dambos, plains and swamps. -

Proposed Sub Sahara Gemstone Exchange Industrial Park
Proposed Sub Sahara Gemstone Exchange Industrial Park Developer SUBSAHARA GEMSTONE EXCHANGE PROJECT MANAGERS PHOENIX MATERIALS LTD 1. Introduction • Project Summary The Sub-Sahara Gemstone Exchange Industrial park is a Multi-Function Economic development Zone located in Ndola, Copperbelt Province along the Ndola-Kabwe Road and approximately 10 Km from Ndola Central Business District. It will be constructed, developed and managed by Phoenix Materials Limited , a wholly Zambian owned Construction and development company. SGE Industrial Park aims to be a modern multi-function economic development park with a variety of facilities including, . Oil Refinery, . Light Manufacturing, . SMEs and MSEs . Container Depot and Dry Port, . high quality multifunctional manufacturing warehouse facilities and Logistics Center. Other support facilities will include . Skills training / incubation Center, . Procurement Services . Shopping Mall, . Office Parks, Hotel and . Residential Developments. Developers SUBSAHARA GEMSTONE EXCHANGE Project Managers PHOENIX MATERIALS LTD 2. Location • Ndola, Zambia . Third Largest City in Zambia . Capital of Copperbelt Province . Population: 495,000 . Has and International Airport . Approx 272Km from Lusaka . Approx 53Km from Kitwe . 10km from DRC Border • SGE Industrial Park, Ndola . Ndola-Kabwe Road – Gateway to the City. Approximately 10Km from CBD . Adjacent to Indeni Oil Refinery . 4Km from Simon Kapwepwe International Airport (former Ndola International Airport ) . Adjacent to Zambia International Trade Fair Developers SUBSAHARA GEMSTONE EXCHANGE Project Managers PHOENIX MATERIALS LTD 3. Site Location • SGE Industrial Park, Ndola . Ndola-Kabwe Road – Gateway to the City. Approximately 10Km from CBD . Adjacent to Indeni Oil Refinery . 4Km from Simon Kapwepwe International Airport (former Ndola International Airport ) . Developers SUBSAHARA GEMSTONE EXCHANGE Project Managers PHOENIX MATERIALS LTD 4. -

Leaders in Urban Transport Planning
Public Disclosure Authorized Leaders in Urban Transport Planning Public Disclosure Authorized WORKSHOP REPORT Public Disclosure Authorized 5 - 11 May 2019 Public Disclosure Authorized Livingstone, Zambia Contents Introduction to LUTP ................................................................................................................. 1 Background ................................................................................................................................ 3 Declining Standards of Public Transport. .............................................................................. 4 Workshop Overview .................................................................................................................. 5 Conceptualization ................................................................................................................... 5 Preparatory Missions .............................................................................................................. 6 Structure and Program ............................................................................................................ 8 Key Partners and Funding ...................................................................................................... 9 Participants ............................................................................................................................. 9 Presenters ............................................................................................................................ -

Zambia Non-Motorised Transport Strategy
Zambia Non-Motorised Transport Strategy Ministry of Transport and Communications United Nations Environment Programme Institute for Transportation and Development Policy July 2019 Contents 1. Introduction ...................................................................................................................................... 3 2. Emerging urban mobility challenges ................................................................................................ 3 3. Assessment of walking & cycling environment ............................................................................... 5 3.1 Footpaths ............................................................................................................................... 5 3.2 Cycle facilities ....................................................................................................................... 7 3.3 Pedestrian crossings ............................................................................................................... 7 3.4 Parking management ............................................................................................................. 8 3.5 Street lighting ........................................................................................................................ 9 3.6 Storm water management .................................................................................................... 10 3.7 Building design ................................................................................................................... -

Central Province General Profile Infographic
ADAPTED BY APRIL 2018 Research and Analysis Department - Designed by Communications ©2018 Policy Monitoring and Research Centre (PMRC) CENTRAL PROVINCE [email protected] www.pmrczambia.com GENERAL PROFILE INFOGRAPHIC SUBSCRIBE NOW: [email protected] GENERAL PROFILE POLITICAL AND ADMINISTRATION PROFILE MAJOR NATURAL RESOURCES LOCATION CONSTITUENCY AGRICULTURE The Province has . Each constituency is represented by (1) elected member Central Province consists of 9.4 million hectares of land, Central province lies at the heart of Zambia. The province shares borders with 16 Constituencies of parliament (MP). CENTRAL about 40% of which is suitable for crop production and the Democratic Republic of Congo and eight other provinces of Zambia with an about 42% for livestock grazing exception of Northern. The Province occupies a total surface area of 108,460km and 16 CONSTITUENCIES PROVINCE is divided into twelve districts namely; Chibombo, Itezhi-tezhi, Kapiri, Shibuyunji FOREST Mposhi, Chitambo, Lunano, Mkushi, Mumbwa, Ngabwe, Chisamba and Serenje with The Province has a total number of 38 forest reserves Kabwe being the provincial capital. The province as at 2010 had a population of LOCAL GOVERNMENT predominantly covered by the Miombo woodland with an 1,332,396 with a gender ratio of 49% male and 51% female. estimated woody plant flora of about 650 species. The Province has (1) Municipality and 12 District Councils. The Mayor/ Council Chairpersons are the political heads of the local government. WILD LIFE 12 DISTRICT COUNCILS The wilderness is characterized by the vastness of unexploited areas, MALE FEMALE such as the Kafue National Park, Itezhi-tezhi dam, the Kafue River and WARDS the wetlands or Kafue flood plains. -
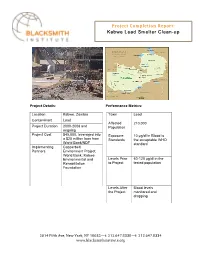
Kabwe Project Completion
F32G-@)!H2BI>-)'2C!J-I23)8!! Kabwe Lead Smelter Clean-up ! !!! !! Project Details: Performance Metrics: Location Kabwe, Zambia Toxin Lead Contaminant Lead Affected 210,000 Project Duration 2000-2008 and Population ongoing Project2014 Fifth Avenue,Cost New York, NY$45 10035,000, | t: 646.742.0200 leveraged | f: 212.779.8044 into | www.blacksmithinstitute.orgExposure 10 !g/dl in Blood is a $20 million loan from Standards the acceptable WHO World Bank/NDF standard Implementing Copperbelt Partners Environment Project; World Bank; Kabwe Environmental and Levels Prior 60-120 !g/dl in the Rehabilitation to Project tested population Foundation Levels After Blood levels the Project monitored and dropping "#$%!&'()*!+,-.!/-0!1234.!/1!$##567)8!"$"9:%;9<55#7(8!"$"9:%;9<55%! 0009=>?@4AB')*'CA)')D)-923E! ! o Background and Scope: Kabwe, the second largest city in Zambia with a population of 300,000, is located about 130km north of the nation's capital, Lusaka. It is one of six towns situated around the "Copperbelt," which was once Zambia's thriving industrial base. In 1902, rich deposits of potentially dangerous lead were discovered in the mine and smelter located in the center of the town. Ore veins with lead concentrations as high as 20 percent have been mined deep into the earth and a smelting operation was set up to process the ore. Mining and smelting operations were running almost continuously up until 1994 without the government addressing the potential danger of lead. The mine and smelter, owned by the now privatized Zambia Consolidated Copper Mines, is no longer operating but has left a city with poison and toxicity from hazardous concentrations of lead in the soil and water.