Exploring the Intracrine Functions of VEGF-A
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Role of Vascular Endothelial Growth Factor Receptor-1 Signaling In
Laboratory Investigation (2015) 95, 456–468 & 2015 USCAP, Inc All rights reserved 0023-6837/15 The role of vascular endothelial growth factor receptor-1 signaling in compensatory contralateral lung growth following unilateral pneumonectomy Yoshio Matsui1, Hideki Amano1,2, Yoshiya Ito3, Koji Eshima4, Hideaki Tamaki5, Fumihiro Ogawa1, Akira Iyoda6, Masafumi Shibuya7, Yuji Kumagai2, Yukitoshi Satoh6 and Masataka Majima1 Compensatory lung growth models have been widely used to investigate alveolization because the remaining lung can be kept intact and volume loss can be controlled. Vascular endothelial growth factor (VEGF) plays an important role in blood formation during lung growth and repair, but the precise mechanisms involved are poorly understood; therefore, the aim of this study was to investigate the role of VEGF signaling in compensatory lung growth. After left pneumonectomy, the right lung weight was higher in VEGF transgenic mice than wild-type (WT) mice. Compensatory lung growth was suppressed significantly in mice injected with a VEGF neutralizing antibody and in VEGF receptor-1 tyrosine kinase-deficient mice (TK À / À mice). The mobilization of progenitor cells expressing VEGFR1 þ cells from bone marrow and the recruitment of these cells to lung tissue were also suppressed in the TK À / À mice.WTmicetransplantedwithbonemarrowfromTKÀ / À transgenic GFP þ mice had significantly lower numbers of GFP þ /aquaporin 5 þ ,GFPþ /surfactant protein A þ ,andGFPþ /VEGFR1 þ cells than WT mice transplanted with bone marrow from WTGFP þ mice. The GFP þ /VEGFR1 þ cells also co-stained for aquaporin 5 and surfactant proteinA.Overall,theseresultssuggest that VEGF signaling contributes to compensatory lung growth by mobilizing VEGFR1 þ cells. -

Ret Oncogene and Thyroid Carcinoma
ndrom Sy es tic & e G n e e n G e f T o Elisei et al., J Genet Syndr Gene Ther 2014, 5:1 Journal of Genetic Syndromes h l e a r n a DOI: 10.4172/2157-7412.1000214 r p u y o J & Gene Therapy ISSN: 2157-7412 Review Article Open Access Ret Oncogene and Thyroid Carcinoma Elisei R, Molinaro E, Agate L, Bottici V, Viola D, Biagini A, Matrone A, Tacito A, Ciampi R, Vivaldi A and Romei C* Endocrine Unit, Department of Clinical and Experimental Medicine, University of Pisa, Italy Abstract Thyroid cancer is a malignant neoplasm that originates from follicular or parafollicular thyroid cells and is categorized as papillary (PTC), follicular (FTC), anaplastic (ATC) or medullary thyroid carcinoma (MTC). The alteration of the Rearranged during trasfection (RET) (proto-oncogene, a gene coding for a tyrosine-kinase receptor involved in the control of cell differentiation and proliferation, has been found to cause PTC and MTC. In particular, RET/PTC rearrangements and RET point mutations are related to PTC and MTC, respectively. Although RET/PTC rearrangements have been identified in both spontaneous and radiation-induced PTC, they occur more frequently in radiation-associated tumors. RET/PTC rearrangements have also been reported in follicular adenomas. Although controversial, correlations between RET/PTC rearrangements, especially RET/PTC3, and a more aggressive phenotype and a more advanced stage have been identified. Germline point mutations in the RET proto-oncogene are associated with nearly all cases of hereditary MTC, and a strict correlation between genotype and phenotype has been demonstrated. -

Serum Levels of VEGF and MCSF in HER2+ / HER2- Breast Cancer Patients with Metronomic Neoadjuvant Chemotherapy Roberto J
Arai et al. Biomarker Research (2018) 6:20 https://doi.org/10.1186/s40364-018-0135-x SHORTREPORT Open Access Serum levels of VEGF and MCSF in HER2+ / HER2- breast cancer patients with metronomic neoadjuvant chemotherapy Roberto J. Arai* , Vanessa Petry, Paulo M. Hoff and Max S. Mano Abstract Metronomic therapy has been gaining importance in the neoadjuvant setting of breast cancer treatment. Its clinical benefits may involve antiangiogenic machinery. Cancer cells induce angiogenesis to support tumor growth by secreting factors, such as vascular endothelial growth factor (VEGF). In breast cancer, Trastuzumab (TZM) based treatment is of key importance and is believed to reduce diameter and volume of blood vessels as well as vascular permeability. Here in we investigated serum levels of angiogenic factors VEGF and MCSF in patients receiving metronomic neoadjuvant therapy with or without TZM. We observed in HER2+ cohort stable levels of MCSF through treatment, whereas VEGF trend was of decreasing levels. In HER2- cohort we observed increasing levels of MCSF and VEGF trend. Overall, HER2+ patients had better pathological response to treatment. These findings suggest that angiogenic pathway may be involved in TZM anti-tumoral effect in the neoadjuvant setting. Keywords: Metronomic chemotherapy, Angiogenesis, Biomarker, Neoadjuvant, Breast cancer Background chemotherapeutic drugs in vitro and in vivo studies [9] Neoadjuvant chemotherapy was initially indicated to con- including rectal carcinomas [10]. Proliferation and/or vert a nonresectable into a resectable lesion [1, 2]. Data on induction of apoptosis of activated endothelial cells the efficacy and safety of metronomic chemotherapy in (ECs) is selectively inhibited as well as inhibition of the neoadjuvant setting for breast cancer (BC) is accumu- migration of EC, increase in the expression of lating and supporting application [3–6]. -

Regulation of Vascular Endothelial Growth Factor Receptor-1 Expression by Specificity Proteins 1, 3, and 4In Pancreatic Cancer Cells
Research Article Regulation of Vascular Endothelial Growth Factor Receptor-1 Expression by Specificity Proteins 1, 3, and 4in Pancreatic Cancer Cells Maen Abdelrahim,1,4 Cheryl H. Baker,4 James L. Abbruzzese,2 David Sheikh-Hamad,3 Shengxi Liu,1 Sung Dae Cho,1 Kyungsil Yoon,1 and Stephen Safe1,5 1Institute of Biosciences and Technology, Texas A&M University Health Science Center; 2Department of Gastrointestinal Medical Oncology, University of Texas M. D. Anderson Cancer Center; 3Division of Nephrology, Department of Medicine, Baylor College of Medicine, Houston, Texas; 4Cancer Research Institute, M. D. Anderson Cancer Center, Orlando, Florida; and 5Department of Veterinary Physiology and Pharmacology, Texas A&M University, College Station, Texas Abstract through their specific interactions with VEGF receptors (VEGFR), Vascular endothelial growth factor receptor-1 (VEGFR1) is which are transmembrane tyrosine kinases and members of the expressed in cancer cell lines and tumors and, in pancreatic PDGF receptor gene family. and colon cancer cells, activation of VEGFR1 is linked to VEGFR1 (Flk-1), VEGFR2(Flt-1/KDR), and VEGFR3 (Flt-4) are the three major receptors for VEGF and related angiogenic factors. increased tumor migration and invasiveness.Tolfenamic acid, The former two receptors are primarily involved in angiogenesis in a nonsteroidal anti-inflammatory drug, decreases Sp protein endothelial cells, whereas VEGFR3 promotes hematopoiesis and expression in Panc-1 and L3.6pl pancreatic cancer cells, and lymphoangiogenesis (2, 3, 7, 8). VEGFR2 plays a critical role in this was accompanied by decreased VEGFR1 protein and angiogenesis; homozygous knockout mice were embryonic lethal mRNA and decreased luciferase activity on cells transfected [gestation day (GD) 8.5–9.0] and this was associated with the with constructs (pVEGFR1) containing VEGFR1 promoter failure to develop blood vessels (9). -

Moguntinones—New Selective Inhibitors for the Treatment of Human Colorectal Cancer
Published OnlineFirst April 17, 2014; DOI: 10.1158/1535-7163.MCT-13-0224 Molecular Cancer Small Molecule Therapeutics Therapeutics Moguntinones—New Selective Inhibitors for the Treatment of Human Colorectal Cancer Annett Maderer1, Stanislav Plutizki2, Jan-Peter Kramb2, Katrin Gopfert€ 1, Monika Linnig1, Katrin Khillimberger1, Christopher Ganser2, Eva Lauermann2, Gerd Dannhardt2, Peter R. Galle1, and Markus Moehler1 Abstract 3-Indolyl and 3-azaindolyl-4-aryl maleimide derivatives, called moguntinones (MOG), have been selected for their ability to inhibit protein kinases associated with angiogenesis and induce apoptosis. Here, we characterize their mode of action and their potential clinical value in human colorectal cancer in vitro and in vivo. MOG-19 and MOG-13 were characterized in vitro using kinase, viability, and apoptosis assays in different human colon cancer (HT-29, HCT-116, Caco-2, and SW480) and normal colon cell lines (CCD-18Co, FHC, and HCoEpiC) alone or in combination with topoisomerase I inhibitors. Intracellular signaling pathways were analyzed by Western blotting. To determine their potential to inhibit tumor growth in vivo, the human HT-29 tumor xenograft model was used. Moguntinones prominently inhibit several protein kinases associated with tumor growth and metastasis. Specific signaling pathways such as GSK3b and mTOR downstream targets b were inhibited with IC50 values in the nanomolar range. GSK3 signaling inhibition was independent of KRAS, BRAF, and PI3KCA mutation status. While moguntinones alone induced apoptosis only in concentra- tions >10 mmol/L, MOG-19 in combination with topoisomerase I inhibitors induced apoptosis synergistically at lower concentrations. Consistent with in vitro data, MOG-19 significantly reduced tumor volume and weight in combination with a topoisomerase I inhibitor in vivo. -

Targeting FGFR/PDGFR/VEGFR Impairs Tumor Growth, Angiogenesis, and Metastasis by Effects on Tumor Cells, Endothelial Cells, and Pericytes in Pancreatic Cancer
Published OnlineFirst September 1, 2011; DOI: 10.1158/1535-7163.MCT-11-0312 Molecular Cancer Preclinical Development Therapeutics Targeting FGFR/PDGFR/VEGFR Impairs Tumor Growth, Angiogenesis, and Metastasis by Effects on Tumor Cells, Endothelial Cells, and Pericytes in Pancreatic Cancer Johannes Taeger1, Christian Moser1, Claus Hellerbrand2, Maria E. Mycielska1, Gabriel Glockzin1, Hans J. Schlitt1, Edward K. Geissler1, Oliver Stoeltzing3, and Sven A. Lang1 Abstract Activation of receptor tyrosine kinases, such as fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and VEGF receptor (VEGFR), has been implicated in tumor progression and metastasis in human pancreatic cancer. In this study, we investigated the effects of TKI258, a tyrosine kinase inhibitor to FGFR, PDGFR, and VEGFR on pancreatic cancer cell lines (HPAF-II, BxPC-3, MiaPaCa2, and L3.6pl), endothelial cells, and vascular smooth muscle cells (VSMC). Results showed that treatment with TKI258 impaired activation of signaling intermediates in pancreatic cancer cells, endothelial cells, and VSMCs, even upon stimulation with FGF-1, FGF-2, VEGF-A, and PDGF-B. Furthermore, blockade of FGFR/PDGFR/VEGFR reduced survivin expression and improved activity of gemcitabine in MiaPaCa2 pancreatic cancer cells. In addition, motility of cancer cells, endothelial cells, and VSMCs was reduced upon treatment with TKI258. In vivo, therapy with TKI258 led to dose-dependent inhibition of subcutaneous (HPAF-II) and orthotopic (L3.6pl) tumor growth. Immunohistochemical analysis revealed effects on tumor cell proliferation [bromodeoxyuridine (BrdUrd)] and tumor vascularization (CD31). Moreover, lymph node metastases were significantly reduced in the orthotopic tumor model when treatment was initiated early with TKI258 (30 mg/kg/d). -

An Intracrine View of Sex Steroids, Immunity, and Metabolic Regulation
Review An intracrine view of sex steroids, immunity, and metabolic regulation Katya B. Rubinow ABSTRACT Background: Over the past two decades, parallel recognition has grown of the importance of both sex steroids and immune activity in metabolic regulation. More recently, these discrete areas have been integrated in studies examining the metabolic effects of sex steroid immunomodulation. Implicit in these studies has been a traditional, endocrine model of sex steroid delivery from the gonads to target cells, including immune cells. Thus, research to date has focused on the metabolic effects of sex steroid receptor signaling in immune cells. This endocrine model, however, overlooks the extensive capacity of immune cells to generate and metabolize sex steroids, enabling the production of sex steroids for intracrine signaling e that is, sex steroid production for signaling within the cell of origin. Intracrine function allows highly cell-autonomous regulation of sex steroid exposure, and sex steroid secretion by immune cells could confer paracrine signaling effects in neighboring cells within metabolic tissues. In this review, immune cell intracrinology will denote sex steroid production within immune cells for either intracrine or paracrine signaling. This intracrine capacity of immune cells has been well established, and prior work has supported its importance in autoimmune disorders, trauma, and cancer. The potential relevance of immune cell intracrine function to the regulation of energy balance, body weight, body composition, and insulin sensitivity has yet to be explored. Scope of review: The following review will detail findings to date regarding the steroidogenic and steroid metabolizing capacity of immune cells, the regulation of immune cell intracrine function, and the biological effects of immune-derived sex steroids, including the clinical relevance of immune cell intracrinology in fields other than metabolism. -

Sulfation Through the Looking Glass—Recent Advances in Sulfotransferase Research for the Curious
The Pharmacogenomics Journal (2002) 2, 297–308 & 2002 Nature Publishing Group All rights reserved 1470-269X/02 $25.00 www.nature.com/tpj REVIEW Sulfation through the looking glass—recent advances in sulfotransferase research for the curious MWH Coughtrie ABSTRACT Members of the cytosolic sulfotransferase (SULT) superfamily catalyse the Department of Molecular & Cellular Pathology, sulfation of a multitude of xenobiotics, hormones and neurotransmitters. University of Dundee, Ninewells Hospital & Humans have at least 10 functional SULT genes, and a number of recent Medical School, Dundee, Scotland, UK advances reviewed here have furthered our understanding of SULT function. Correspondence: Analysis of expression patterns has shown that sulfotransferases are highly MWH Coughtrie, Department of expressed in the fetus, and SULTs may in fact be a major detoxification Molecular & Cellular Pathology, University enzyme system in the developing human. The X-ray crystal structures of of Dundee, Ninewells Hospital and three SULTs have been solved and combined with mutagenesis experiments Medical School, Dundee DD1 9SY, Scotland, UK. and molecular modelling, they have provided the first clues as to the factors Tel: +44 (0)1382 632510 that govern the unique substrate specificities of some of these enzymes. In Fax: +44 (0)1382 640320 the future these and other studies will facilitate prediction of the fate of E-mail: [email protected] chemicals metabolised by sulfation. Variation in sulfation capacity may be important in determining an individual’s response to xenobiotics, and there has been an explosion in information on sulfotransferase polymorphisms and their functional consequences, including the influence of SULT1A1 genotype on susceptibility to colorectal and breast cancer. -
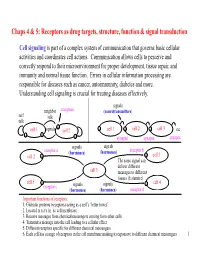
Chaps 4 & 5: Receptors As Drug Targets, Structure, Function & Signal Transduction
Chaps 4 & 5: Receptors as drug targets, structure, function & signal transduction Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. Communication allows cells to perceive and correctly respond to their microenvironment for proper development, tissue repair, and immunity and normal tissue function. Errors in cellular information processing are responsible for diseases such as cancer, autoimmunity, diabetes and more. Understanding cell signaling is crucial for treating diseases effectively. signals neighbor receptors (neurotransmitters) self talk talk cell 1 signals cell 2 cell 1 cell 2 cell 3 etc. synapse synapse synapse signals signals receptor a (hormones) (hormones) receptor b cell 2 cell 3 The same signal can deliver different cell 1 messages to different tissues (histamine). cell 5 cell 4 receptor c signals signals (hormones) (hormones) receptor d Important functions of receptors: 1. Globular proteins (receptors) acting as a cell’s ‘letter boxes’ 2. Located mostly in the cell membrane 3. Receive messages from chemical messengers coming from other cells 4. Transmit a message into the cell leading to a cellular effect 5. Different receptors specific for different chemical messengers 6. Each cell has a range of receptors in the cell membrane making it responsive to different chemical messengers 1 © Oxford University Press, 2013 Signals can be divided into the 5 catagories below. Signal carriers (S) have to reach the proper receptors (R) for the message to be recieved. Intracrine signals are produced by the target cell that stay within the target cell. cell a S = signal S R R = receptor Autocrine signals are produced by the target cell, are secreted, and affect the target cell itself via receptors. -

Unveiling the Molecular Mechanisms Regulating the Activation of the Erbb Family Receptors at Atomic Resolution Through Molecular Modeling and Simulations
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations Spring 2011 Unveiling the Molecular Mechanisms Regulating the Activation of the ErbB Family Receptors at Atomic Resolution through Molecular Modeling and Simulations Andrew Shih University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biophysics Commons, Other Biomedical Engineering and Bioengineering Commons, and the Structural Biology Commons Recommended Citation Shih, Andrew, "Unveiling the Molecular Mechanisms Regulating the Activation of the ErbB Family Receptors at Atomic Resolution through Molecular Modeling and Simulations" (2011). Publicly Accessible Penn Dissertations. 302. https://repository.upenn.edu/edissertations/302 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/302 For more information, please contact [email protected]. Unveiling the Molecular Mechanisms Regulating the Activation of the ErbB Family Receptors at Atomic Resolution through Molecular Modeling and Simulations Abstract The EGFR/ErbB/HER family of kinases contains four homologous receptor tyrosine kinases that are important regulatory elements in key signaling pathways. To elucidate the atomistic mechanisms of dimerization-dependent activation in the ErbB family, we have performed molecular dynamics simulations of the intracellular kinase domains of the four members of the ErbB family (those with known kinase activity), namely EGFR, ErbB2 (HER2) -

The Role of Signaling Pathways in the Development and Treatment of Hepatocellular Carcinoma
Oncogene (2010) 29, 4989–5005 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 www.nature.com/onc REVIEW The role of signaling pathways in the development and treatment of hepatocellular carcinoma S Whittaker1,2, R Marais3 and AX Zhu4 1Dana-Farber Cancer Institute, Boston, MA, USA; 2The Broad Institute, Cambridge, MA, USA; 3Institute of Cancer Research, London, UK and 4Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA Hepatocellular carcinoma (HCC) is a highly prevalent, malignancy in adults (Pons-Renedo and Llovet, 2003). treatment-resistant malignancy with a multifaceted mole- For the vast majority of patients, HCC is a late cular pathogenesis. Current evidence indicates that during complication of chronic liver disease, and as such, is hepatocarcinogenesis, two main pathogenic mechanisms often associated with cirrhosis. The main risk factors for prevail: (1) cirrhosis associated with hepatic regeneration the development of HCC include infection with hepatitis after tissue damage caused by hepatitis infection, toxins B virus (HBV) or hepatitis C virus (HCV). Hepatitis (for example, alcohol or aflatoxin) or metabolic influ- infection is believed to be the main etiologic factor in ences, and (2) mutations occurring in single or multiple 480% of cases (Anzola, 2004). Other risk factors oncogenes or tumor suppressor genes. Both mechanisms include excessive alcohol consumption, nonalcoholic have been linked with alterations in several important steatohepatitis, autoimmune hepatitis, primary biliary cellular signaling pathways. These pathways are of cirrhosis, exposure to environmental carcinogens (parti- interest from a therapeutic perspective, because targeting cularly aflatoxin B) and the presence of various genetic them may help to reverse, delay or prevent tumorigenesis. -

Structural and Mechanistic Insights Into VEGF Receptor 3 Ligand Binding and Activation
Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation Veli-Matti Leppänena,1, Denis Tvorogova,1, Kaisa Kiskob, Andrea E. Protab, Michael Jeltscha, Andrey Anisimova, Sandra Markovic-Muellerb, Edward Stuttfeldb,c, Kenneth N. Goldied, Kurt Ballmer-Hoferb, and Kari Alitaloa,2 aWihuri Research Institute and Translational Cancer Biology Program, Institute for Molecular Medicine Finland and Helsinki University Central Hospital, Biomedicum Helsinki, University of Helsinki, 00014 Helsinki, Finland; bLaboratory of Biomolecular Research, Paul Scherrer Institute, CH-5232 Villigen PSI, Switzerland; cStructural Biology and Biophysics, Biozentrum, University of Basel, CH-4056 Basel, Switzerland; and dCenter for Cellular Imaging and NanoAnalytics, Biozentrum, University of Basel, CH-4056 Basel, Switzerland Edited by Napoleone Ferrara, University of California at San Diego, La Jolla, CA, and approved June 26, 2013 (received for review January 23, 2013) Vascular endothelial growth factors (VEGFs) and their receptors binding, VEGFRs use predominantly D2, with D3 providing ad- (VEGFRs) are key drivers of blood and lymph vessel formation in ditional binding affinity (16–18). In addition, D1 is required for development, but also in several pathological processes. VEGF-C VEGFR-3 ligand binding, but the exact role of this domain re- signaling through VEGFR-3 promotes lymphangiogenesis, which is mains elusive (19, 20). VEGFRs and the closely related type III a clinically relevant target for treating lymphatic insufficiency and RTKs are activated by ligand-induced dimerization of the extra- for blocking tumor angiogenesis and metastasis. The extracellular cellular domain, followed by tyrosine autophosphorylation of the domain of VEGFRs consists of seven Ig homology domains; intracellular kinase domain to generate downstream signaling domains 1–3 (D1-3) are responsible for ligand binding, and the (21).