Research Article INTERACTION of SELECTED ANTHOCYANINS
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
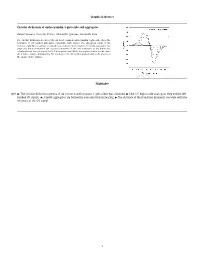
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins
CROATICA CHEMICA ACTA CCACAA 80 (1) 29¿34 (2007) ISSN-0011-1643 CCA-3135 Original Scientific Paper Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins Andréia A. de Lima, Eliana M. Sussuchi, and Wagner F. De Giovani* Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto – SP, Brazil RECEIVED JUNE 26, 2006; REVISED SEPTEMBER 29, 2006; ACCEPTED OCTOBER 27, 2006 Electrochemical properties of delphinidin, cyanidin, pelargonidin, kuromanin and callistephin were investigated by cyclic and differential pulse voltammetries at different pH values and also in methanol. On the basis of oxidation potentials, the order of antioxidant activity for antho- cyanidins is delphinidin > cyanidin > pelargonidin. Oxidation peaks for anthocyanins (kuro- manin and callistephin) are shifted to more positive potentials compared to anthocyanidins (delphinidin, cyanidin and pelargonidin). Oxidation peak currents are linearly dependent on the Keywords square root of the scan rate, which is typical of a diffusion controlled electrochemical process. anthocyanins Antioxidant activities of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydra- anthocyanidins zyl (DPPH) radical-scavenging method and they are directly related to their redox potential antioxidants values. The order of the antioxidant activity is delphinidin > cyanidin > pelargonidin > kuro- electrochemical properties manin > callistephin. INTRODUCTION pharmacological properties that render them interesting potential cancer chemopreventive agents.9–11 Because Anthocyanins are one of the main classes of flavonoids anthocyanins are widely consumed, finding out addi- in red wines. They have been pointed out as the molecu- tional biological activities related to these compounds lar entities that are most likely responsible for what has would be of great interest. -

(Roxb.) Craib and Kadsura Coccinea (Lem.) AC
foods Article Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Varittha Sritalahareuthai 1, Piya Temviriyanukul 1,2 , Nattira On-nom 1,2 , Somsri Charoenkiatkul 1 and Uthaiwan Suttisansanee 1,2,* 1 Institute of Nutrition, Mahidol University, Salaya, Phuttamonthon, Nakhon Pathom 73170, Thailand; [email protected] (V.S.); [email protected] (P.T.); [email protected] (N.O.-n.); [email protected] (S.C.) 2 Food and Nutrition Academic and Research Cluster, Institute of Nutrition, Mahidol University, Salaya, Phuttamonthon, Nakhon Pathom 73170, Thailand * Correspondence: [email protected]; Tel.: +66-(0)2800-2380 (ext. 422) Received: 4 August 2020; Accepted: 31 August 2020; Published: 2 September 2020 Abstract: Kadsura spp. in the Schisandraceae family are woody vine plants, which produce edible red fruits that are rich in nutrients and antioxidant activities. Despite their valuable food applications, Kadsura spp. are only able to grow naturally in the forest, and reproduction handled by botanists is still in progress with a very low growth rate. Subsequently, Kadsura spp. were listed as endangered species by the International Union for Conservation of Nature and Natural Resources (IUCN) in 2011. Two different Kadsura spp., including Kadsura coccinea (Lem.) A.C. Sm. and Kadsura heteroclita (Roxb.) Craib, are mostly found in northern Thailand. These rare, wild fruits are unrecognizable to outsiders, and there have only been limited investigations into its biological properties. This study, therefore, aimed to comparatively investigate the phenolic profiles, antioxidant activities, and inhibitory activities against the key enzymes involved in diabetes (α-glucosidase and α-amylase) and Alzheimer’s disease (acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-secretase 1 (BACE-1)) in different fruit parts (exocarp, mesocarp (edible part), seed, and core) of Kadsura coccinea (Lem.) A.C. -

Callistephin Enhances the Protective Effects of Isoflurane on Microglial Injury Through Downregulation of Inflammation and Apoptosis
802 MOLECULAR MEDICINE REPORTS 20: 802-812, 2019 Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis LILI ZHAO, SHIBIAO CHEN, TIANYIN LIU, XIUHONG WANG, HAIJIN HUANG and WEICHENG LIU Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received June 18, 2018; Accepted March 15, 2019 DOI: 10.3892/mmr.2019.10282 Abstract. Microglia are the major immune cells in the central enhanced the effects of isoflurane. Callistephin may therefore nervous system. Microglial activation can be beneficial or constitute a candidate drug agent that may target inflammatory detrimental depending on the stimuli and the physiopathological and growth regulatory signaling pathways, thus ameliorating environment. Microglial activation is involved in a variety certain aspects of neurodegenerative diseases. of neurodegenerative disorders. Different anesthetic agents have exhibited diverse effects on microglial activation and Introduction the engulfment process. The anthocyanin callistephin has been demonstrated to have antioxidant and anti‑inflammatory Microglial cells are the major immune cell in the central properties, and these were assessed in the present study, with a nervous system (CNS), responding against types of endog- focus on its effect on microglial activation. Mouse microglial enous and exogenous stimuli, including infection by bacteria, cells C8-4B were treated with 100 ng/µl lipopolysaccharide viruses, prions and β-amyloid plaques (1). Microglia are (LPS) and 1 ng/µl interferon-γ. Cells were subsequently treated activated upon exposure to different stimuli and, depending with 2% isoflurane, 100 µM callistephin or both. LPS promoted on the environmental context, this may be beneficial or detri- apoptosis in C8-B4 cells, and this was reduced following mental to the functionality and physiology of the CNS (2). -

(Lapageria Rosea) C
J. Chil. Chem. Soc., 54, Nº 2 (2009) ANTHOCYANINS THAT CONFER CHARACTERISTIC COLOR TO RED COPIHUE FLOWERS (LAPAGERIA ROSEA) C. VERGARA1, D. VON BAER1*, I. HERMOSÍN2, A. RUIZ1, M.A. HITSCHFELD1, N. CASTILLO2 AND C. MARDONES1. 1 Universidad de Concepción, Departamento de Análisis Instrumental, Facultad de Farmacia, Casilla 160 – C, Concepción, Chile. 2 Universidad de Castilla-La Mancha, Escuela Universitaria de Ingeniería Técnica Agrícola, Ronda de Calatrava 7, 13071 Ciudad Real, España. (Received: January 7, 2009 - Accepted: February 25, 2009) ABSTRACT The Copihue (Lapageria rosea), also known as the Chilean bellflower, is the national flower of Chile and is the only species in the genus Lapageria. The copihue’s tepals are commonly red, with white or pink being less common. The red color of the copihue has been glorified in legends, poems and popular songs. The present work studies the pigments that confer red copihues their characteristic color. The principal types of cyanidin present in red copihue’s tepals are cyanidin-3-O-rhamnosylglucoside, followed by cyanidin-3-O-glucoside, and while only the latter is detected in pink tepals and neither one are detected in white flowers. Based on the obtained results by HPLC-ESI-MSn and HPLC-DAD, it is concluded that rhamnosyl- and glucosyl-derivatives of cyanidin, which present respectively an absorption maximum at 518 and 516 nm, confer the characteristic red color to red copihues. Furthermore, glycosilated cyanidin derivatives, pigments derived from other anthocyanidins, were not detected in red copihue flowers even when they are present in other red flowering plants. Keywords: flower, copihue, Lapageria rosea, anthocyanins, cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside. -

Characterisation of Bioactive Compounds in Berries from Plants Grown Under Innovative Photovoltaic Greenhouses
Journal of Berry Research 8 (2018) 55–69 55 DOI:10.3233/JBR-170258 IOS Press Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses Federica Blandoa,∗, Carmela Gerardia, Massimiliano Rennab,c, Sergio Castellanod and Francesco Seriob aInstitute of Sciences of Food Production (ISPA), CNR, Lecce, Italy bInstitute of Sciences of Food Production (ISPA), CNR, Bari, Italy cDepartment of Agricultural and Environmental Science, University of Bari Aldo Moro, Bari, Italy dDepartment of Science of Agriculture, Food and Environment, (SAFE) University of Foggia, Foggia, Italy Received 2 July 2017; accepted 1 November 2017 Abstract. BACKGROUND: Bioactive compounds, mainly polyphenols, present in berries, are thought to be responsible for the health benefits of these fruit. Therefore, it is worthwhile to define the optimal environmental conditions to maximise their polyphenol content. OBJECTIVE: With the aim to define the optimal conditions for berry cultivation in an innovative environment, red rasp- berry, wild strawberry and blackberry plants were grown in a traditional greenhouse in comparison with two photovoltaic greenhouses with different shading area. METHODS: Hydroalcoholic extracts of ripe berries were evaluated by HPLC analysis, for their anthocyanins, organic acids and sugar contents. Moreover, phenolic content (by the Folin-Ciocalteu assay) and antioxidant activity (by the Trolox equivalent antioxidant capacity-TEAC assay) were assayed on the same berry extracts. RESULTS: Total anthocyanins, -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

First-Pass Metabolism of Polyphenols from Selected Berries: a High-Throughput Bioanalytical Approach
antioxidants Article First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach Francisco J. Olivas-Aguirre 1,*, Sandra Mendoza 2, Emilio Alvarez-Parrilla 3 , Gustavo A. Gonzalez-Aguilar 4 , Monica A. Villegas-Ochoa 4 , Jael T.J. Quintero-Vargas 1 and Abraham Wall-Medrano 3,* 1 Departamento de Ciencias de la Salud, Universidad de Sonora (Campus Cajeme), Blvd Bordo Nuevo s/n, Ejido Providencia, Cd, Obregón 85199, Mexico; [email protected] 2 Departamento de Investigación y Posgrado en Alimentos (PROPAC), Facultad de Química, Universidad Autónoma de Querétaro, Cerro de las Campanas s/n, Santiago de Querétaro 76010, Mexico; [email protected] 3 Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo s/n, Ciudad Juárez 32310, Mexico; [email protected] 4 Centro de Investigación en Alimentación y Desarrollo, A.C. (CIAD), Carretera a Ejido La Victoria, Km. 0.6, Hermosillo 83304, Mexico; [email protected] (G.A.G.-A.); [email protected] (M.A.V.-O.) * Correspondence: [email protected] (F.J.O.-A.); [email protected] (A.W.-M.) Received: 29 March 2020; Accepted: 11 April 2020; Published: 13 April 2020 Abstract: Small berries are rich in polyphenols whose first-pass metabolism may alter their ultimate physiological effects. The antioxidant capacity and polyphenol profile of three freeze-dried berries (blackberry, raspberry, Red Globe grape) were measured and their apparent permeability (Papp) and first-pass biotransformation were tracked with an ex vivo bioanalytical system [everted gut sac (rat) + three detection methods: spectrophotometry, HPLC-ESI-QTOF-MS, differential pulse voltammetry (DPV)]. -

Anthocyanin Metabolites in Human Urine and Serum
Downloaded from https://www.cambridge.org/core British Journal of Nutrition (2004), 91, 933–942 DOI: 10.1079/BJN20041126 q Minister of Public Works and Government Services Canada 2003 Anthocyanin metabolites in human urine and serum . IP address: 1,2 1,2 2 3 Colin D. Kay , G. Mazza *, Bruce J. Holub and Jian Wang 170.106.34.90 1Pacific Agri-Food Research Center, Agriculture and Agri-Food Canada, 4200 Hwy 97 Summerland, British Columbia, Canada V0H 1Z0 2 Department of Human Biology and Nutritional Sciences, University of Guelph, Ontario, Canada , on 3 Calgary Laboratory, Canadian Food Inspection Agency, Calgary, Alberta, Canada 02 Oct 2021 at 01:38:57 (Received 26 September 2003 – Revised 2 February 2004 – Accepted 11 February 2004) In the present study we investigated the metabolic conversion of cyanidin glycosides in human subjects using solid-phase extraction, HPLC–diode array detector, MS, GC, and enzymic techniques. Volunteers consumed approximately 20 g chokeberry extract containing , subject to the Cambridge Core terms of use, available at 1·3 g cyanidin 3-glycosides (899 mg cyanidin 3-galactoside, 321 mg cyanidin 3-arabinoside, 51 mg cyanidin 3-xyloside and 50 mg cyanidin 3-glucoside). Blood samples were drawn at 0, 0·5, 1, and 2 h post-consumption of the extract. Urine samples were also collected at 0, 4–5, and 22–24 h. We have confirmed that human subjects have the capacity to metabolise cyanidin 3-glycosides, as we observed at least ten individual anthocyanin metabolites in the urine and serum. Average concentrations of anthocyanins and anthocyanin metabolites in the urine reached levels of 17·9 (range 14·9–20·9) mmol/l within 5 h post-consumption and persisted in 24 h urine samples at levels of 12·1 (range 11·1–13·0) nmol/l. -

Effect of Different Anthocyanidin Glucosides on Lutein Uptake By
molecules Article Effect of Different Anthocyanidin Glucosides on Lutein Uptake by Caco-2 Cells, and Their Combined Activities on Anti-Oxidation and Anti-Inflammation In Vitro and Ex Vivo Minh Anh Thu Phan 1,*, Martin Bucknall 2 and Jayashree Arcot 1,* ID 1 Food and Health Cluster, School of Chemical Engineering, UNSW, Sydney, NSW 2052, Australia 2 Mark Wainwright Analytical Centre, UNSW, Sydney, NSW 2052, Australia; [email protected] * Correspondence: [email protected] (M.A.T.P.); [email protected] (J.A.); Tel.: +61-2-9385-5360 (J.A.) Academic Editors: Natália Martins and Derek J. McPhee Received: 23 July 2018; Accepted: 11 August 2018; Published: 14 August 2018 Abstract: The interactive effects on anti-oxidation and anti-inflammation of lutein combined with each of the six common anthocyanidin glucosides were studied in both chemical and cellular systems. The combined phytochemicals showed an antagonism in the inhibition of lipid oxidation in a liposomal membrane, but showed an additive effect on cellular antioxidant activity in Caco-2 cells. Lutein was an active lipoxygenase inhibitor at 2–12 µM while anthocyanins were inactive. The concentration of lutein when it was used in combination with anthocyanins was 25–54% higher than when lutein was used alone (i.e., IC50 = 1.2 µM) to induce 50% of lipoxygenase inhibition. Only the combination of lutein with malvidin-3-glucoside showed anti-inflammatory synergy in the suppression of interleukin-8, and the synergy was seen at all three ratios tested. Some mixtures, however, showed anti-inflammatory antagonism. The presence of anthocyanins (5–7.5 µM) did not affect lutein uptake (2.5–5 µM) by Caco-2 cells. -

Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines During Simulated Digestion
molecules Article Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines during Simulated Digestion Tomasz Tarko * , Aleksandra Duda-Chodak and Agata Soszka Department of Fermentation Technology and Microbiology, Faculty of Food Technology, University of Agriculture in Krakow, ul. Balicka 122, 30-149 Krakow, Poland; [email protected] (A.D.-C.); [email protected] (A.S.) * Correspondence: [email protected]; Tel.: +48-12-6624792 Academic Editor: Urszula Gawlik-Dziki Received: 5 November 2020; Accepted: 24 November 2020; Published: 27 November 2020 Abstract: The content of polyphenols (total phenolic content (TPC)) and the antioxidant activity (AOX) of food products depend on the raw materials used and the technological processes in operation, but transformations of these compounds in the digestive tract are very important. The aim of this study was to determine the TPC, profile of polyphenols, and AOX of apple and blackcurrant musts and wines in order to evaluate the changes occurring in a simulated human digestive system. The research material consisted of apples and blackcurrant, from which musts and fruit wines were obtained. All samples were subjected to three-stage digestion in a simulated human digestive system and then analyzed for the following: TPC (Folin–Ciocalteu method) and profile (HPLC), AOX (method with 2,20-azino-bis (3-ethylbenzothiazoline-6-sulfonic) acid (ABTS) radical), and for the wines also total extract, volatile acidity (International Organization of Vine and Wine (OIV) method), and sugar profile (HPLC). The antioxidant activity of fruit wines is directly related to the total polyphenol content. Phenolic compounds were transformed during all digestive stages, which led to the formation of compounds with higher antioxidant capacity. -

Inertsearch for LC
InertSearchTM for LC Inertsil ® Applications Analysis of Bilberry extract (InertSustain C18) Data No. LA904-0871 14 1 2 3 5 4 8 7 15 6 11 12 10 9 13 0 1020304050 Time (min) Conditions Analyte: System : LC800 HPLC system Bilberry powder (1.25 mg/mL) Column : InertSustain C18 (5 μm, 250 x 4.6 mm I.D) 1. Delphinidin-3-O-galactoside Column Cat. No. : 5020-07346 2. Delphinidin-3-O-glucoside Eluent : A) H2O/CH3CN/CH3OH/HCOOH = 40/22.5/22.5/10, v/v/v 3. Cyanidin-3-O-galactoside B) H2O/HCOOH =90/10, v/v 4. Delphinidin-3-O-arabinoside A/B = 7/93 -35 min- 25/75 -10 min- 65/35 5. Cyanidin-3-O-glucoside -1 min- 100/0 -4 min- 100/0, v/v 6. Petunidin-3-O-galactoside (Mixed by a gradient mixer) 7. Cyanidin-3-O-grabinoside Flow Rate : 1.0 mL/min 8. Petunidin-3-O-glucoside Col. Temp. : 30 ℃ 9. Peonidin-3-O-galactoside Detection : VIS 535 nm (LC800 UV Detector) 10. Petunidin-3-O-arabinoside Injection Vol. : 10 μL 11. Peonidin-3-O-glucoside Sample : Powdered bilberry extract (USP) 12. Malvidin-3-O-galactoside 13. Peonidin-3-O-arabinoside 14. Malvidin-3-O-glucoside 15. Malvidin-3-O-arabinoside LC LA904 InertSustain C18 HP D Delphinidin-3-glucoside chloride タデルフィニジン-3-オー-グルコシドクロリド Delphinidin-3-glucoside chloride Delphinidin-3-O-glucoside chloride Myrtillin chloride デルフィニジン-3-オー-グルコシドクロリド デルフィニジン-3-グルコシドクロリド ミルチリンクロリド 6906-38-3 LC LA904 InertSustain C18 HP D Delphinidin-3-O-galactoside chloride タデルフィニジン-3-オー-ガラクトシドクロリド Delphinidin-3-O-galactoside chloride Delphinidin-3-galactoside chloride デルフィニジン-3-オー-ガラクトシドクロリド デルフィニジン-3-ガラクトシドクロリド 28500-00-7 LC LA904 InertSustain