Flower Color Inheritance of Rhynchostylis Gigantea (Lindl.) Ridl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
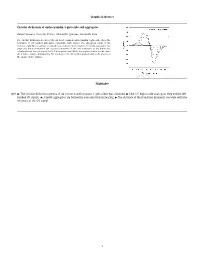
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Minireview Article the Current Research Status of Endangered
Minireview Article The Current Research Status of Endangered Rhynchostylis retusa (L.) Blume: A Review ABSTRACT Orchids bear the crown of highly evolved ornamental floral specialization in the plant kingdom, but have poor medicinal background. Further, Rhynchostylis retusa (L.) Blume is much less studied plant species among Orchidaceae due to its overexploitation in the wild. This epiphytic herb belongs to the tropical areas and kept under endangered category by CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) that points out its abated natural population. In this sense, the aim is to document the past and present researches upon R. retusa highlighting the traditional and remedial uses, and to ignite the conservation awareness among the people. Keywords: Conservation, medicines, mythology, patents, taxonomy, tissue culture. 1. INTRODUCTION Orchidaceae (monocotyledons) are one of the largest families of the plant world extending from tropics to alpines along with a boon of astonishing beauty in their flowers [1]. Orchids are the richest among angiosperms with diverse species number (approximately >25,000) having epiphytes (more abundant, 73 %), lithophytes and ground plants [2,3]. Fossil records of orchids date back to 100 million years ago [4]. Actually the term ‘orchid’ coming from ‘orchis’ (Greek word) meaning testicle was given by Theophrastus (372-286 B.C.), who reported the use of orchid roots as aphrodiasic. Despite of huge family size, orchids are largely exploited and traded for their ornamental flower diversity but less attention is paid towards their remedial properties. According to Reinikka, the first evidence of the use of orchids as medicines comes from Shênnung’s Materia Medica in 28th century B.C. -

Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins
CROATICA CHEMICA ACTA CCACAA 80 (1) 29¿34 (2007) ISSN-0011-1643 CCA-3135 Original Scientific Paper Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins Andréia A. de Lima, Eliana M. Sussuchi, and Wagner F. De Giovani* Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto – SP, Brazil RECEIVED JUNE 26, 2006; REVISED SEPTEMBER 29, 2006; ACCEPTED OCTOBER 27, 2006 Electrochemical properties of delphinidin, cyanidin, pelargonidin, kuromanin and callistephin were investigated by cyclic and differential pulse voltammetries at different pH values and also in methanol. On the basis of oxidation potentials, the order of antioxidant activity for antho- cyanidins is delphinidin > cyanidin > pelargonidin. Oxidation peaks for anthocyanins (kuro- manin and callistephin) are shifted to more positive potentials compared to anthocyanidins (delphinidin, cyanidin and pelargonidin). Oxidation peak currents are linearly dependent on the Keywords square root of the scan rate, which is typical of a diffusion controlled electrochemical process. anthocyanins Antioxidant activities of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydra- anthocyanidins zyl (DPPH) radical-scavenging method and they are directly related to their redox potential antioxidants values. The order of the antioxidant activity is delphinidin > cyanidin > pelargonidin > kuro- electrochemical properties manin > callistephin. INTRODUCTION pharmacological properties that render them interesting potential cancer chemopreventive agents.9–11 Because Anthocyanins are one of the main classes of flavonoids anthocyanins are widely consumed, finding out addi- in red wines. They have been pointed out as the molecu- tional biological activities related to these compounds lar entities that are most likely responsible for what has would be of great interest. -

An Endangered Orchid
Asian Journal of Conservation Biology, December 2020. Vol. 9 No. 2, pp. 275-279 AJCB: FP0144 ISSN 2278-7666 ©TCRP Foundation 2020 Asymbiotic Seed Germination, Mass propagation and Conservation of Fox-tail orchid, Rhynchostylis retusa L. Blume: An endangered orchid Kumari Fonseka* Department of Crop Science, Faculty of Agriculture, University of Ruhuna, Sri Lanka (Received: May 25, 2020; Revised: June 23, 2020; Accepted: July 22, 2020) ABSTRACT Experiments were carried out to identify the correct maturity stage for in-vitro seed germination and to develop an in-vitro protocol for mass propagation of Rhynchostylis retusa. After hand pollination of the flowers the length, girth and color changes of the pod were observed in weekly intervals in order to determine the morpho- logical indicators for maturity. In-vitro seed germination was done 3, 4, 5, 6 and 7 months after pollination on nine media viz 1. Murashige and Skoog medium (MS) 2. MS with PVP (2g/L) 3. MS with charcoal (2g/L) 4. Knudson C medium (KNC) 5.KNC with PVP (2g/L) 6. KNC with charcoal (2g/L) 7. KNC with banana extracts (75g/L) 8.Vacin and Went medium (V&W) 9. V&W with banana extract (75g/L) and coconut water (150ml/L). Results revealed that six months after pollination was the best stage for in-vitro seed germination of R. retusa. Significant germination was observed only on MS with PVP, MS with charcoal, KNC with banana extracts and V&W with banana extracts and coconut water (p ≤ 0.05). KNC with banana extracts and V&W with banana extracts and coconut water gave 100% seed germination. -

Birth of an Orchid Hartley Creating Vanda William Catherine
Stefania Birth of an orchid Hartley Creating Vanda William Catherine Key words Orchids are beautiful plants and they have great Orchids that are pollinated by flies and midges orchids commercial value. So how are new varieties release a scent of algae, yeast, crustaceans or rotting meat. Flies land on the orchid, slip and slide plant breeding developed? Stefania Hartley explains. off into a large bag formed by the lower petal. The fly can only escape by squeezing through a narrow hybrid ust imagine. After spending many months in tunnel, rubbing off any pollen received from other the tropical forest, where you have managed to pollination J flowers and receiving a new load. avoid deadly diseases and cannibalistic tribes, you Most orchids grow in tropical regions but they are setting off home. But your ship catches fire also occur in temperate regions and are present in and all its precious cargo is lost. Luckily, you have every continent except Antarctica. Some orchids survived. All you wish for is to reach home. You grow on trees (without being parasitic, using the send a telegram to your employer to inform him of tree for support) and they are called epiphytic (from the disaster and then you wait for instructions. His Greek: epi = on; phytos = plant). They have thick reply is sharp and cruel: “Turn back – collect more.” roots surrounded by a layer of dead cells called Obediently you oblige and start all over again. velamen which absorbs water. Geophytic orchids This is what happened to Wilhelm Micholitz, one live on the ground (geos = Earth). -

Callistephin Enhances the Protective Effects of Isoflurane on Microglial Injury Through Downregulation of Inflammation and Apoptosis
802 MOLECULAR MEDICINE REPORTS 20: 802-812, 2019 Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis LILI ZHAO, SHIBIAO CHEN, TIANYIN LIU, XIUHONG WANG, HAIJIN HUANG and WEICHENG LIU Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received June 18, 2018; Accepted March 15, 2019 DOI: 10.3892/mmr.2019.10282 Abstract. Microglia are the major immune cells in the central enhanced the effects of isoflurane. Callistephin may therefore nervous system. Microglial activation can be beneficial or constitute a candidate drug agent that may target inflammatory detrimental depending on the stimuli and the physiopathological and growth regulatory signaling pathways, thus ameliorating environment. Microglial activation is involved in a variety certain aspects of neurodegenerative diseases. of neurodegenerative disorders. Different anesthetic agents have exhibited diverse effects on microglial activation and Introduction the engulfment process. The anthocyanin callistephin has been demonstrated to have antioxidant and anti‑inflammatory Microglial cells are the major immune cell in the central properties, and these were assessed in the present study, with a nervous system (CNS), responding against types of endog- focus on its effect on microglial activation. Mouse microglial enous and exogenous stimuli, including infection by bacteria, cells C8-4B were treated with 100 ng/µl lipopolysaccharide viruses, prions and β-amyloid plaques (1). Microglia are (LPS) and 1 ng/µl interferon-γ. Cells were subsequently treated activated upon exposure to different stimuli and, depending with 2% isoflurane, 100 µM callistephin or both. LPS promoted on the environmental context, this may be beneficial or detri- apoptosis in C8-B4 cells, and this was reduced following mental to the functionality and physiology of the CNS (2). -

The Intergeneric Crossing of Phalaenopsis Sp. and Vanda Tricolor
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sebelas Maret Institutional Repository Journal of Biotechnology and Biodiversity, March 2010; 1(1): 32 -36 ISSN: 2087-0183 RESEARCH The intergeneric crossing of Phalaenopsis sp. and Vanda tricolor Sri Hartatia* aDepartment of Agronomy, Faculty of Agriculture, Sebelas Maret University, Jl. Ir. Sutami no 36A, Surakarta 57126, Indonesia Received : 5 August 2009 Accepted: 27 August 2009 Abstract To study the intergeneric crossing between orchids of Phalaenopsis sp. and Vanda tricolor, three species of Phalaenopsis sp. (Phalaenopsis Joane Kileup June, Phalaenopsis Pinlong Cinderella, and (Phal. Fortune Buddha x Phal. Princess Kaiulani) were crossed reciprocally with Vanda tricolor in time-different value (within the first, second and third week after full opened flower). The crossing of Phalaenopsis sp. and Vanda tricolor was compatible, and the use of Phalaenopsis sp. as male parent had better probability in producing fruits rather than the opposite. The crossing which was done at the first and the second weeks after blooming produced more fruit than the crossing at the third week after blooming, even though it did not affect the success of crossing, time of fruit formation, and duration of fruit hanging. Key words: Intergeneric Crossing, Orchids, Phalaenopsis sp., Vanda tricolor INTRODUCTION One strategy to make a new-hybrid compatible to Doritis pulcherrima var. cultivars of orchids is by crossing between the Champornensis (Hartati, unpublished results). orchid-parents having different characters. To assess the effect of time of crossing (week Orchid hobbyist usually prever to collect after blooming) in order to make new hybrid hybrid resulted from crossing orchids, orchids with more attractive flower characters because the hybrided orchids have more to the fruit formation in, Phalaenopsis sp. -

Traditional Therapeutic Uses of Some Indigenous Orchids of Bangladesh
® Medicinal and Aromatic Plant Science and Biotechnology ©2009 Global Science Books Traditional Therapeutic Uses of Some Indigenous Orchids of Bangladesh Mohammad Musharof Hossain* Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh Correspondence : * [email protected] ABSTRACT The traditional therapeutic uses of some indigenous orchids of Bangladesh are described in this paper. Terrestrial (11) and epiphytic (18) orchids, 29 in total, are used by Bangladeshi rural and tribal people for the treatment of nearly 45 different diseases and ailments. Roots, tubers, pseudobulbs, stems, leaves and even whole plants are used. Some herbal preparations have miraculous curative properties. Unfortunately, these preparations have not typically been subjected to the precise scientific clarification and standardization which are consequently required for clinical implementations. Some of the orchids are endangered due to over-exploitation and habitat destruction. Conservation strategies for orchids and further pharmacological studies on traditional medicines are suggested. _____________________________________________________________________________________________________________ Keywords: astavarga, conservation, ethnomedicine, herbal remedies, rasna INTRODUCTION the underground tuber of Orchis latifolia is used in the drug ‘munjatak’ pacifying cough (Khasim and Rao 1999). The Orchidaceae is the largest and most evolved family of the leaves of Vanda roxburghii have been prescribed in the flowering plants, consisting of 2500 to 35,000 species bel- ancient ‘Sanskrit’ literature for external application in rheu- onging to 750-800 genera (Dressler 1993). They are found matism, ear infections, fractures and diseases of nervous in virtually all regions around the world except the icy system. Besides these, other orchids used in local systems Antarctica, but their greatest diversity occurs in tropical and of medicine are Cleisostoma williamsonii (for bone frac- sub-tropical regions. -

Review Article Organic Compounds: Contents and Their Role in Improving Seed Germination and Protocorm Development in Orchids
Hindawi International Journal of Agronomy Volume 2020, Article ID 2795108, 12 pages https://doi.org/10.1155/2020/2795108 Review Article Organic Compounds: Contents and Their Role in Improving Seed Germination and Protocorm Development in Orchids Edy Setiti Wida Utami and Sucipto Hariyanto Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia Correspondence should be addressed to Sucipto Hariyanto; [email protected] Received 26 January 2020; Revised 9 May 2020; Accepted 23 May 2020; Published 11 June 2020 Academic Editor: Isabel Marques Copyright © 2020 Edy Setiti Wida Utami and Sucipto Hariyanto. ,is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In nature, orchid seed germination is obligatory following infection by mycorrhizal fungi, which supplies the developing embryo with water, carbohydrates, vitamins, and minerals, causing the seeds to germinate relatively slowly and at a low germination rate. ,e nonsymbiotic germination of orchid seeds found in 1922 is applicable to in vitro propagation. ,e success of seed germination in vitro is influenced by supplementation with organic compounds. Here, we review the scientific literature in terms of the contents and role of organic supplements in promoting seed germination, protocorm development, and seedling growth in orchids. We systematically collected information from scientific literature databases including Scopus, Google Scholar, and ProQuest, as well as published books and conference proceedings. Various organic compounds, i.e., coconut water (CW), peptone (P), banana homogenate (BH), potato homogenate (PH), chitosan (CHT), tomato juice (TJ), and yeast extract (YE), can promote seed germination and growth and development of various orchids. -

Characterisation of Bioactive Compounds in Berries from Plants Grown Under Innovative Photovoltaic Greenhouses
Journal of Berry Research 8 (2018) 55–69 55 DOI:10.3233/JBR-170258 IOS Press Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses Federica Blandoa,∗, Carmela Gerardia, Massimiliano Rennab,c, Sergio Castellanod and Francesco Seriob aInstitute of Sciences of Food Production (ISPA), CNR, Lecce, Italy bInstitute of Sciences of Food Production (ISPA), CNR, Bari, Italy cDepartment of Agricultural and Environmental Science, University of Bari Aldo Moro, Bari, Italy dDepartment of Science of Agriculture, Food and Environment, (SAFE) University of Foggia, Foggia, Italy Received 2 July 2017; accepted 1 November 2017 Abstract. BACKGROUND: Bioactive compounds, mainly polyphenols, present in berries, are thought to be responsible for the health benefits of these fruit. Therefore, it is worthwhile to define the optimal environmental conditions to maximise their polyphenol content. OBJECTIVE: With the aim to define the optimal conditions for berry cultivation in an innovative environment, red rasp- berry, wild strawberry and blackberry plants were grown in a traditional greenhouse in comparison with two photovoltaic greenhouses with different shading area. METHODS: Hydroalcoholic extracts of ripe berries were evaluated by HPLC analysis, for their anthocyanins, organic acids and sugar contents. Moreover, phenolic content (by the Folin-Ciocalteu assay) and antioxidant activity (by the Trolox equivalent antioxidant capacity-TEAC assay) were assayed on the same berry extracts. RESULTS: Total anthocyanins, -

First-Pass Metabolism of Polyphenols from Selected Berries: a High-Throughput Bioanalytical Approach
antioxidants Article First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach Francisco J. Olivas-Aguirre 1,*, Sandra Mendoza 2, Emilio Alvarez-Parrilla 3 , Gustavo A. Gonzalez-Aguilar 4 , Monica A. Villegas-Ochoa 4 , Jael T.J. Quintero-Vargas 1 and Abraham Wall-Medrano 3,* 1 Departamento de Ciencias de la Salud, Universidad de Sonora (Campus Cajeme), Blvd Bordo Nuevo s/n, Ejido Providencia, Cd, Obregón 85199, Mexico; [email protected] 2 Departamento de Investigación y Posgrado en Alimentos (PROPAC), Facultad de Química, Universidad Autónoma de Querétaro, Cerro de las Campanas s/n, Santiago de Querétaro 76010, Mexico; [email protected] 3 Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo s/n, Ciudad Juárez 32310, Mexico; [email protected] 4 Centro de Investigación en Alimentación y Desarrollo, A.C. (CIAD), Carretera a Ejido La Victoria, Km. 0.6, Hermosillo 83304, Mexico; [email protected] (G.A.G.-A.); [email protected] (M.A.V.-O.) * Correspondence: [email protected] (F.J.O.-A.); [email protected] (A.W.-M.) Received: 29 March 2020; Accepted: 11 April 2020; Published: 13 April 2020 Abstract: Small berries are rich in polyphenols whose first-pass metabolism may alter their ultimate physiological effects. The antioxidant capacity and polyphenol profile of three freeze-dried berries (blackberry, raspberry, Red Globe grape) were measured and their apparent permeability (Papp) and first-pass biotransformation were tracked with an ex vivo bioanalytical system [everted gut sac (rat) + three detection methods: spectrophotometry, HPLC-ESI-QTOF-MS, differential pulse voltammetry (DPV)]. -

Rhynchostylis Gigantea
Growing Notes Rhynchostylis gigantea rink-oh-STY-lis jy-GAN-tee-ah Rhynchostylis gigantea has definitely become a collector’s item in recent years with ever more unusual color forms being introduced into the marketplace. The unmistakeable spicy fragrance is consistent across this genus, whether the species blooms in the winter or summer. One species, Rhy. retusa, is referred to as the “fox tail” orchid because of its long tapering inflorescence. The inflorescence of Rhynchostylis gigantea is somewhat shorter, the actual length being determined by genetics and culture of the individual plant. There is something exotic and magical about walking into a winter greenhouse and encountering the warm fragrance of Rhy. gigantea. It leaves a lasting impression one looks forward to year after year. This orchid prefers the warm humid climate of Southeast Asia and ranges from Hainan, China through Myanmar, Laos, Thailand, Viet Nam, Malaysia and into the Philippines. Rhy. gigantea was discovered in Burma (Myanmar) by Wallich and described as Saccolabium giganteum by Lindley in 1833. Ridley transferred it to Rhynchostylis in 1896 (J. Linn. Soc., Bot. 32: 356). Over the years there have been numerous varietal names applied to different color forms but they are all of horticultural use only. At this time, the Kew Monocot Checklist recognizes only two subspecies with Rhynchostylis gigantea subsp. violacea (Lindl.) Christenson, being endemic to the Philippines. Although this is indeed a warm-growing orchid, it is tolerant of a wider range of light conditions than other Vandaceous orchids making it more acessible to hobbyists. We like to grow it less bright than our Vanda’s and ascocendas..