Predation in the Marine Fossil Record Studies, Data, Recognition
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Muricidae, from Palk Strait, Southeast Coast of India
Nature Environment and Pollution Technology Vol. 8 No. 1 pp. 63-68 2009 An International Quarterly Scientific Journal Original Research Paper New Record of Muricanthus kuesterianus (Tapparone-Canefri, 1875) Family: Muricidae, from Palk Strait, Southeast Coast of India C. Stella and C. Raghunathan* Department of Oceanography and Coastal Area Studies, Alagappa University, Thondi-623 409, Ramnad district, Tamil Nadu, India *Zoological Survey of India, Andaman and Nicobar Regional Station, Haddo, Port Blair-744 102, Andaman & Nicobar Islands, India Key Words: ABSTRACT Gastropoda The present study reported the occurrence of Muricanthus kuesterianus in the Palk Muricidae Strait region of southeast coast of India as a first hand record. The detailed description Muricanthus kuesterianus of this species has been given with the comparison of its close resembled species Chicoreus virgineus Chicoreus virgineus. INTRODUCTION Muricidae, the largest and varied taxonomic family among marine gastropods has small to large predatory sea snails in the Order Neogastropoda. At least 1,000 species of muricids under numerous subfamilies are known. Many muricids have unusual shells which are considered attractive by shell collectors. The spire and body whorl of the muricids are often ornamental with knobs, tubercules, ribbing or spines. Muricids have episodic growth which means that the shell grows in spurts, remain- ing in the same size for a while before rapidly growing to the next size stage resulting in a series of varices on each whorl. Most species of muricids are carnivorous, feeding on other gastropods, bivalves and barnacles. In March 2007, during the course of faunistic surveys along the Palk Strait region of southeast coast of India (Fig. -

Has Its Charm
VOL. XXXV NO.1 JANUARY. 1987 NEW SERIES 325 TREE SNAILERS! IT'S TIME TO UNITE Has Its Charm ByRON KNIGHT LORENGAU, MANUS ISLAND, PAPUA NEW By TWILA BRATCHER GUINEA - We amateur specialists in terrestrial Unspoiled Rowley Shoals lies in the easternmost molluscs are subject to some special handicaps. Not Indian Ocean, about 180 miles west of Broome, only are we scattered around the world and fre- Western Australia. The occasionalcharter boats that visit the area have been doing so only recently, and quently isolated in the much larger body of marine foreign fishing fleets are not permitted there. shell enthusiasts, but we suffer from a seeming shor- The only important scientific work on Rowley tage of literature. Many of us have nowhere to turn Shoalswas done by groups from the Western Aus- tralian Museum in Perth. for advice, assistance and comparative material. I was a participant in a recent privately organized Professionals interested in the pulmonates have trip to the Rowley Shoals. Lynn Funkhouser, the easier access to literature, of course, but they face group leader, is an accomplished professional a more subtle handicap. That is their wide separa- underwater photographer and president of the tion from the enthusiasts in the field who tradi- Chicago Shell Club. Others were JeanetteRidley, underwater photographer from Seattle; Philip and tionally supply the scientists with the specimens, Heidrun Faulconer, underwaterphotographers from data and puzzlers that are the feed stock of the San Diego, John and Mary Poble, underwater technical literature. All of us - scientists and photographersfrom Nebraska, my sister Billee Dil- amateurs, professionals and beginners - are the worth Brown, a docent at Scripps Aquarium in La McNally's Trophies Photo: Schoenberg Jolla, and myself. -
Wec01's SSSS Fossils Test 2019
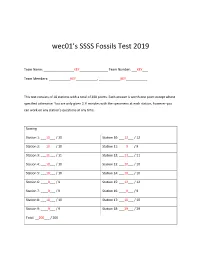
wec01’s SSSS Fossils Test 2019 Team Name: _________________KEY________________ Team Number: ___KEY___ Team Members: ____________KEY____________, ____________KEY____________ This test consists of 18 stations with a total of 200 points. Each answer is worth one point except where specified otherwise. You are only given 2 ½ minutes with the specimens at each station, however you can work on any station’s questions at any time. Scoring Station 1: ___10___ / 10 Station 10: ___12___ / 12 Station 2: ___10___ / 10 Station 11: ____9___ / 9 Station 3: ___11___ / 11 Station 12: ___11___ / 11 Station 4: ___10___ / 10 Station 13: ___10___ / 10 Station 5: ___10___ / 10 Station 14: ___10___ / 10 Station 6: ____9___ / 9 Station 15: ___12___ / 12 Station 7: ____9___ / 9 Station 16: ____9___ / 9 Station 8: ___10___ / 10 Station 17: ___10___ / 10 Station 9: ____9___ / 9 Station 18: ___29___ / 29 Total: __200___ / 200 Team Number: _KEY_ Station 1: Dinosaurs (10 pt) 1. Identify the genus of specimen A Tyrannosaurus (1 pt) 2. Identify the genus of specimen B Stegosaurus (1 pt) 3. Identify the genus of specimen C Allosaurus (1 pt) 4. Which specimen(s) (A, B, or C) are A, C (1 pt) Saurischians? 5. Which two specimens (A, B, or C) lived at B, C (1 pt) the same time? 6. Identify the genus of specimen D Velociraptor (1 pt) 7. Identify the genus of specimen E Coelophysis (1 pt) 8. Which specimen (D or E) is commonly E (1 pt) found in Ghost Ranch, New Mexico? 9. Which specimen (A, B, C, D, or E) would D (1 pt) specimen F have been found on? 10. -

Brittle-Star Mass Occurrence on a Late Cretaceous Methane Seep from South Dakota, USA Received: 16 May 2018 Ben Thuy1, Neil H
www.nature.com/scientificreports OPEN Brittle-star mass occurrence on a Late Cretaceous methane seep from South Dakota, USA Received: 16 May 2018 Ben Thuy1, Neil H. Landman2, Neal L. Larson3 & Lea D. Numberger-Thuy1 Accepted: 29 May 2018 Articulated brittle stars are rare fossils because the skeleton rapidly disintegrates after death and only Published: xx xx xxxx fossilises intact under special conditions. Here, we describe an extraordinary mass occurrence of the ophiacanthid ophiuroid Brezinacantha tolis gen. et sp. nov., preserved as articulated skeletons from an upper Campanian (Late Cretaceous) methane seep of South Dakota. It is uniquely the frst fossil case of a seep-associated ophiuroid. The articulated skeletons overlie centimeter-thick accumulations of dissociated skeletal parts, suggesting lifetime densities of approximately 1000 individuals per m2, persisting at that particular location for several generations. The ophiuroid skeletons on top of the occurrence were preserved intact most probably because of increased methane seepage, killing the individuals and inducing rapid cementation, rather than due to storm-induced burial or slumping. The mass occurrence described herein is an unambiguous case of an autochthonous, dense ophiuroid community that persisted at a particular spot for some time. Thus, it represents a true fossil equivalent of a recent ophiuroid dense bed, unlike other cases that were used in the past to substantiate the claim of a mid-Mesozoic predation-induced decline of ophiuroid dense beds. Brittle stars, or ophiuroids, are among the most abundant and widespread components of the marine benthos, occurring at all depths and latitudes of the world oceans1. Most of the time, however, ophiuroids tend to live a cryptic life hidden under rocks, inside sponges, epizoic on corals or buried in the mud (e.g.2) to such a point that their real abundance is rarely appreciated at frst sight. -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Deep-Sea Fauna of the European Seas: an Annotated Species Check-List Of
Invertebrate Zoology, 2014, 11(1): 134–155 © INVERTEBRATE ZOOLOGY, 2014 Deep-sea fauna of European seas: An annotated species check-list of benthic invertebrates living deeper than 2000 m in the seas bordering Europe. Gastropoda Alexander V. Sysoev Zoological Museum, Moscow State University, Bol’shaya Nikitskaya ul., 6, Moscow, 125009, Russia. E-mail: [email protected] ABSTRACT: An annotated check-list is given of Gastropoda species occurring deeper than 2000 m in the seas bordering Europe. The check-list is based on published data. The check- list includes 221 species. For each species data on localities in European seas and general species distribution are provided. Station data are presented separately in the present thematic issue. How to cite this article: Sysoev A.V. 2014. Deep-sea fauna of European seas: An annotated species check-list of benthic invertebrates living deeper than 2000 m in the seas bordering Europe. Gastropoda // Invert. Zool. Vol.11. No.1. P.134–155. KEY WORDS: deep-sea fauna, European seas, Gastropoda. Глубоководная фауна европейских морей: аннотированный список видов донных беспозвоночных, обитающих глубже 2000 м в морях, окружающих Европу. Gastropoda А.В. Сысоев Зоологический музей МГУ им. М.В. Ломоносова, ул. Большая Никитская, 6, Москва 125009, Россия. E-mail: [email protected] РЕЗЮМЕ: Приводится аннотированный список видов Gastropoda, обитающих глуб- же 2000 м в морях, окружающих Европу. Список основан на опубликованных данных. Список насчитывает 221 вид. Для каждого вида приведены данные о нахождениях в европейских морях и сведения о распространении. Данные о станци- ях приводятся в отдельном разделе настоящего тематического выпуска. Как цитировать эту статью: Sysoev A.V. -

Functional Morphology of the Vertebral Column in Remingtonocetus (Mammalia, Cetacea) and the Evolution of Aquatic Locomotion in Early Archaeocetes
Functional Morphology of the Vertebral Column in Remingtonocetus (Mammalia, Cetacea) and the Evolution of Aquatic Locomotion in Early Archaeocetes by Ryan Matthew Bebej A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Ecology and Evolutionary Biology) in The University of Michigan 2011 Doctoral Committee: Professor Philip D. Gingerich, Co-Chair Professor Philip Myers, Co-Chair Professor Daniel C. Fisher Professor Paul W. Webb © Ryan Matthew Bebej 2011 To my wonderful wife Melissa, for her infinite love and support ii Acknowledgments First, I would like to thank each of my committee members. I will be forever grateful to my primary mentor, Philip D. Gingerich, for providing me the opportunity of a lifetime, studying the very organisms that sparked my interest in evolution and paleontology in the first place. His encouragement, patience, instruction, and advice have been instrumental in my development as a scholar, and his dedication to his craft has instilled in me the importance of doing careful and solid research. I am extremely grateful to Philip Myers, who graciously consented to be my co-advisor and co-chair early in my career and guided me through some of the most stressful aspects of life as a Ph.D. student (e.g., preliminary examinations). I also thank Paul W. Webb, for his novel thoughts about living in and moving through water, and Daniel C. Fisher, for his insights into functional morphology, 3D modeling, and mammalian paleobiology. My research was almost entirely predicated on cetacean fossils collected through a collaboration of the University of Michigan and the Geological Survey of Pakistan before my arrival in Ann Arbor. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Durham Research Online
Durham Research Online Deposited in DRO: 23 May 2017 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Betts, Marissa J. and Paterson, John R. and Jago, James B. and Jacquet, Sarah M. and Skovsted, Christian B. and Topper, Timothy P. and Brock, Glenn A. (2017) 'Global correlation of the early Cambrian of South Australia : shelly fauna of the Dailyatia odyssei Zone.', Gondwana research., 46 . pp. 240-279. Further information on publisher's website: https://doi.org/10.1016/j.gr.2017.02.007 Publisher's copyright statement: c 2017 This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Accepted Manuscript Global correlation of the early Cambrian of South Australia: Shelly fauna of the Dailyatia odyssei Zone Marissa J. -

By C. M. Yonge, D.Se. University of Bristol
453 EVOLUTION OF CILIARY FEEDING IN THE PROSOBRANCHIA, WITH AN ACCOUNT OF FEEDING IN GAPULUS UNGAR/GUS By C. M. Yonge, D.Se. University of Bristol (Text-figs. 1-6) CONTENTS PAGE Introduction 453 Rejection Currents in the Mantle Cavity of the Prosobranchia 453 Evolution of Ciliary Feeding 455 Vermetus novae-hollandiae . 456 Crepidula fornicata and other Calyptraeidae 457 Capulus ungaricus 459 Modification of gill filaments 461 Discussion. 465 Summary 467 References. 468 INTRODUCTION Ciliary feeding, of such widespread occurrence in the Lamellibranchia, is confined in the Gastropoda to a few scattered groups. In freshwater Pul- monata, such as Limnaea, cilia on the foot assist in feeding when the animal is creeping suspended from the surface film (Brockmeier, 1898). Thecoso- matous Pteropoda feed exclusively by the aid of cilia on the unpaired middle lobe and the paired side lobes of the foot, and an evolutionary series- Cavolinia-Cymbulia-Gleba-can be traced in which there is a progressive elaboration in the perfection of this mechanism and an accompanying reduc- tion in the buccal mass and associated structures handed down from carni- vorous ancestors (Yonge, 1926). Only in the few prosobranchs which have acquired ciliary feeding mechanisms do these represent a modification of the ctenidia as in the Lamellibranchia. They also, as it is the aim of this paper to show, represent a modification of the rejection currents present in the mantle cavity of typical prosobranchs. REJECTION CURRENTS IN THE MANTLE CAVITY OF THE PROSOBRANCHIA In typical Prosobranchia a respiratory current, created by the beating of the lateral cilia on the gill filaments, is drawn into the mantle cavity by way of the inhalent opening (frequently prolonged into a siphon, e.g. -

Phylogenomic Resolution of the Class Ophiuroidea Unlocks a Global Microfossil Record
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 24, 1874–1879, August 18, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2014.06.060 Report Phylogenomic Resolution of the Class Ophiuroidea Unlocks a Global Microfossil Record Timothy D. O’Hara,1,* Andrew F. Hugall,1 Ben Thuy,2 We used a phylogenomic approach to address these defi- and Adnan Moussalli1 ciencies, obtaining 52 de novo ophiuroid transcriptomes and 1Museum Victoria, GPO Box 666, Melbourne, VIC 3001, three from other echinoderm classes, in addition to three pub- Australia lically available transcriptomes and three genomes (see Table 2Section Pale´ ontologie, Muse´ e National d’Histoire Naturelle du S1 available online). Twenty-nine of the ophiuroid transcrip- Luxembourg, 24 Rue Mu¨ nster, 2160 Luxembourg tomes were obtained from deep-sea (>500 m) samples, to ensure adequate taxonomic representation across the class. We identified and aligned 425 orthologous genes that could Summary be annotated using available sea urchin, hemichordate, or actinopterygian genomes (Table S2). After trimming, we used Our understanding of the origin, evolution, and biogeog- 102,143 amino acid positions to reconstruct a phylogeny of raphy of seafloor fauna is limited because we have insuf- the Echinodermata, with a focus on the ophiuroids (Figure 1). ficient spatial and temporal data to resolve underlying The resulting sequence matrix is 85% complete (Figure S1) processes [1]. The abundance and wide distribution of mod- and is the largest genetic data set ever assembled to analyze ern and disarticulated fossil Ophiuroidea [2], including brittle phylogenetic relationships within echinoderms.