By C. M. Yonge, D.Se. University of Bristol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

MOLLUSCA Nudibranchs, Pteropods, Gastropods, Bivalves, Chitons, Octopus
UNDERWATER FIELD GUIDE TO ROSS ISLAND & MCMURDO SOUND, ANTARCTICA: MOLLUSCA nudibranchs, pteropods, gastropods, bivalves, chitons, octopus Peter Brueggeman Photographs: Steve Alexander, Rod Budd/Antarctica New Zealand, Peter Brueggeman, Kirsten Carlson/National Science Foundation, Canadian Museum of Nature (Kathleen Conlan), Shawn Harper, Luke Hunt, Henry Kaiser, Mike Lucibella/National Science Foundation, Adam G Marsh, Jim Mastro, Bruce A Miller, Eva Philipp, Rob Robbins, Steve Rupp/National Science Foundation, Dirk Schories, M Dale Stokes, and Norbert Wu The National Science Foundation's Office of Polar Programs sponsored Norbert Wu on an Artist's and Writer's Grant project, in which Peter Brueggeman participated. One outcome from Wu's endeavor is this Field Guide, which builds upon principal photography by Norbert Wu, with photos from other photographers, who are credited on their photographs and above. This Field Guide is intended to facilitate underwater/topside field identification from visual characters. Organisms were identified from photographs with no specimen collection, and there can be some uncertainty in identifications solely from photographs. © 1998+; text © Peter Brueggeman; photographs © Steve Alexander, Rod Budd/Antarctica New Zealand Pictorial Collection 159687 & 159713, 2001-2002, Peter Brueggeman, Kirsten Carlson/National Science Foundation, Canadian Museum of Nature (Kathleen Conlan), Shawn Harper, Luke Hunt, Henry Kaiser, Mike Lucibella/National Science Foundation, Adam G Marsh, Jim Mastro, Bruce A Miller, Eva -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Marine Boring Bivalve Mollusks from Isla Margarita, Venezuela
ISSN 0738-9388 247 Volume: 49 THE FESTIVUS ISSUE 3 Marine boring bivalve mollusks from Isla Margarita, Venezuela Marcel Velásquez 1 1 Museum National d’Histoire Naturelle, Sorbonne Universites, 43 Rue Cuvier, F-75231 Paris, France; [email protected] Paul Valentich-Scott 2 2 Santa Barbara Museum of Natural History, Santa Barbara, California, 93105, USA; [email protected] Juan Carlos Capelo 3 3 Estación de Investigaciones Marinas de Margarita. Fundación La Salle de Ciencias Naturales. Apartado 144 Porlama,. Isla de Margarita, Venezuela. ABSTRACT Marine endolithic and wood-boring bivalve mollusks living in rocks, corals, wood, and shells were surveyed on the Caribbean coast of Venezuela at Isla Margarita between 2004 and 2008. These surveys were supplemented with boring mollusk data from malacological collections in Venezuelan museums. A total of 571 individuals, corresponding to 3 orders, 4 families, 15 genera, and 20 species were identified and analyzed. The species with the widest distribution were: Leiosolenus aristatus which was found in 14 of the 24 localities, followed by Leiosolenus bisulcatus and Choristodon robustus, found in eight and six localities, respectively. The remaining species had low densities in the region, being collected in only one to four of the localities sampled. The total number of species reported here represents 68% of the boring mollusks that have been documented in Venezuelan coastal waters. This study represents the first work focused exclusively on the examination of the cryptofaunal mollusks of Isla Margarita, Venezuela. KEY WORDS Shipworms, cryptofauna, Teredinidae, Pholadidae, Gastrochaenidae, Mytilidae, Petricolidae, Margarita Island, Isla Margarita Venezuela, boring bivalves, endolithic. INTRODUCTION The lithophagans (Mytilidae) are among the Bivalve mollusks from a range of families have more recognized boring mollusks. -

Faunal Change and Bathymetric Diversity Gradient in Deep-Sea Prosobranchs from Northeastern Atlantic
Biodiversity and Conservation (2006) Ó Springer 2006 DOI 10.1007/s10531-005-1344-9 -1 Faunal change and bathymetric diversity gradient in deep-sea prosobranchs from Northeastern Atlantic CELIA OLABARRIA1,2 1Southampton Oceanography Centre, DEEPSEAS Benthic Biology Group, Empress Dock, South- ampton SO14 3ZH, United Kingdom; 2Present address: Departamento de Ecoloxı´a e Bioloxı´a Animal, Area Ecoloxı´a, Universidad de Vigo, Campus Lagoas-Marcosende, 36310 Vigo (Pontevedra), Spain (e-mail: [email protected]; phone: +34-986-812587; fax: +34-986-812556) Received 6 January 2005; accepted in revised form 11 July 2005 Key words: Deep sea, Diversity, Faunal turnover, Northeastern Atlantic, Porcupine Abyssal Plain, Porcupine Seabight, Prosobranchs Abstract. Despite the plethora of studies, geographic patterns of diversity in deep sea remain subject of speculation. This study considers a large dataset to examine the faunal change and depth-diversity gradient of prosobranch molluscs in the Porcupine Seabight and adjacent Abyssal Plain (NE Atlantic). Rates of species succession (addition and loss) increased rapidly with increasing depth and indicated four possible areas of faunal turnover at about 700, 1600, 2800 and 4100 m. Depth was a significant predictor of diversity, explaining nearly a quarter the variance. There was a pattern of decreasing diversity downslope from 250 m to 1500–1600 m, followed by an increase to high values at about 4000 m and then again, a fall to 4915 m. Processes causing diversity patterns of prosobranchs in the Porcupine Seabight and adjacent Abyssal Plain are likely to differ in magnitude or type, from those operating in other Atlantic areas. Introduction An increasing focus for biodiversity research in the deep sea has been to test for the existence of large-scale gradients in the diversity of marine soft- sediment fauna in deep sea (e.g. -

The Limpet Form in Gastropods: Evolution, Distribution, and Implications for the Comparative Study of History
UC Davis UC Davis Previously Published Works Title The limpet form in gastropods: Evolution, distribution, and implications for the comparative study of history Permalink https://escholarship.org/uc/item/8p93f8z8 Journal Biological Journal of the Linnean Society, 120(1) ISSN 0024-4066 Author Vermeij, GJ Publication Date 2017 DOI 10.1111/bij.12883 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Biological Journal of the Linnean Society, 2016, , – . With 1 figure. Biological Journal of the Linnean Society, 2017, 120 , 22–37. With 1 figures 2 G. J. VERMEIJ A B The limpet form in gastropods: evolution, distribution, and implications for the comparative study of history GEERAT J. VERMEIJ* Department of Earth and Planetary Science, University of California, Davis, Davis, CA,USA C D Received 19 April 2015; revised 30 June 2016; accepted for publication 30 June 2016 The limpet form – a cap-shaped or slipper-shaped univalved shell – convergently evolved in many gastropod lineages, but questions remain about when, how often, and under which circumstances it originated. Except for some predation-resistant limpets in shallow-water marine environments, limpets are not well adapted to intense competition and predation, leading to the prediction that they originated in refugial habitats where exposure to predators and competitors is low. A survey of fossil and living limpets indicates that the limpet form evolved independently in at least 54 lineages, with particularly frequent origins in early-diverging gastropod clades, as well as in Neritimorpha and Heterobranchia. There are at least 14 origins in freshwater and 10 in the deep sea, E F with known times ranging from the Cambrian to the Neogene. -
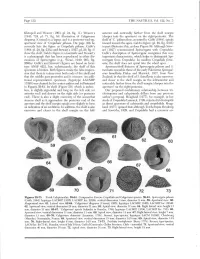
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave. -

Terra Nova Bay, Ross Sea
MEASURE 14 - ANNEX Management Plan for Antarctic Specially Protected Area No 161 TERRA NOVA BAY, ROSS SEA 1. Description values to be protected A coastal marine area encompassing 29.4km2 between Adélie Cove and Tethys Bay, Terra Nova Bay, is proposed as an Antarctic Specially Protected Area (ASPA) by Italy on the grounds that it is an important littoral area for well-established and long-term scientific investigations. The Area is confined to a narrow strip of waters extending approximately 9.4km in length immediately to the south of the Mario Zucchelli Station (MZS) and up to a maximum of 7km from the shore. No marine resource harvesting has been, is currently, or is planned to be, conducted within the Area, nor in the immediate surrounding vicinity. The site typically remains ice-free in summer, which is rare for coastal areas in the Ross Sea region, making it an ideal and accessible site for research into the near-shore benthic communities of the region. Extensive marine ecological research has been carried out at Terra Nova Bay since 1986/87, contributing substantially to our understanding of these communities which had not previously been well-described. High diversity at both species and community levels make this Area of high ecological and scientific value. Studies have revealed a complex array of species assemblages, often co-existing in mosaics (Cattaneo-Vietti, 1991; Sarà et al., 1992; Cattaneo-Vietti et al., 1997; 2000b; 2000c; Gambi et al., 1997; Cantone et al., 2000). There exist assemblages with high species richness and complex functioning, such as the sponge and anthozoan communities, alongside loosely structured, low diversity assemblages. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

Caenogastropoda
13 Caenogastropoda Winston F. Ponder, Donald J. Colgan, John M. Healy, Alexander Nützel, Luiz R. L. Simone, and Ellen E. Strong Caenogastropods comprise about 60% of living Many caenogastropods are well-known gastropod species and include a large number marine snails and include the Littorinidae (peri- of ecologically and commercially important winkles), Cypraeidae (cowries), Cerithiidae (creep- marine families. They have undergone an ers), Calyptraeidae (slipper limpets), Tonnidae extraordinary adaptive radiation, resulting in (tuns), Cassidae (helmet shells), Ranellidae (tri- considerable morphological, ecological, physi- tons), Strombidae (strombs), Naticidae (moon ological, and behavioral diversity. There is a snails), Muricidae (rock shells, oyster drills, etc.), wide array of often convergent shell morpholo- Volutidae (balers, etc.), Mitridae (miters), Buccin- gies (Figure 13.1), with the typically coiled shell idae (whelks), Terebridae (augers), and Conidae being tall-spired to globose or fl attened, with (cones). There are also well-known freshwater some uncoiled or limpet-like and others with families such as the Viviparidae, Thiaridae, and the shells reduced or, rarely, lost. There are Hydrobiidae and a few terrestrial groups, nota- also considerable modifi cations to the head- bly the Cyclophoroidea. foot and mantle through the group (Figure 13.2) Although there are no reliable estimates and major dietary specializations. It is our aim of named species, living caenogastropods are in this chapter to review the phylogeny of this one of the most diverse metazoan clades. Most group, with emphasis on the areas of expertise families are marine, and many (e.g., Strombidae, of the authors. Cypraeidae, Ovulidae, Cerithiopsidae, Triphori- The fi rst records of undisputed caenogastro- dae, Olividae, Mitridae, Costellariidae, Tereb- pods are from the middle and upper Paleozoic, ridae, Turridae, Conidae) have large numbers and there were signifi cant radiations during the of tropical taxa. -

Proceedings of the United States National Museum
a Proceedings of the United States National Museum SMITHSONIAN INSTITUTION • WASHINGTON, D.C. Volume 121 1967 Number 3579 VALID ZOOLOGICAL NAMES OF THE PORTLAND CATALOGUE By Harald a. Rehder Research Curator, Division of Mollusks Introduction An outstanding patroness of the arts and sciences in eighteenth- century England was Lady Margaret Cavendish Bentinck, Duchess of Portland, wife of William, Second Duke of Portland. At Bulstrode in Buckinghamshire, magnificent summer residence of the Dukes of Portland, and in her London house in Whitehall, Lady Margaret— widow for the last 23 years of her life— entertained gentlemen in- terested in her extensive collection of natural history and objets d'art. Among these visitors were Sir Joseph Banks and Daniel Solander, pupil of Linnaeus. As her own particular interest was in conchology, she received from both of these men many specimens of shells gathered on Captain Cook's voyages. Apparently Solander spent considerable time working on the conchological collection, for his manuscript on descriptions of new shells was based largely on the "Portland Museum." When Lady Margaret died in 1785, her "Museum" was sold at auction. The task of preparing the collection for sale and compiling the sales catalogue fell to the Reverend John Lightfoot (1735-1788). For many years librarian and chaplain to the Duchess and scientif- 1 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. 121 ically inclined with a special leaning toward botany and conchology, he was well acquainted with the collection. It is not surprising he went to considerable trouble to give names and figure references to so many of the mollusks and other invertebrates that he listed. -

Gastropoda, Capulidae)
1 2 3 SUSPENSION FEEDING AND KLEPTOPARASITISM WITHIN THE 4 GENUS TRICHOTROPIS (GASTROPODA, CAPULIDAE) 5 6 ERIKA V. IYENGAR 7 Department of Biology, Muhlenberg College, 2400 W. Chew St., Allentown, PA 18104, USA. 8 9 10 RUNNING HEAD: SUSPENSION FEEDING & KLEPTOPARASITISM IN TRICHOTROPIS 11 12 Telephone, fax, email: 13 Correspondence: E.V. Iyengar; e-mail: [email protected] 14 15 phone: (484) 664-3731; FAX: (484) 664-3002 16 17 Suspension feeding and kleptoparasitism in Trichotropis 1 ABSTRACT 2 The marine gastropod Trichotropis cancellata is a facultative kleptoparasite, either 3 suspension feeding or parasitically stealing food from tube-dwelling polychaete worms. To 4 determine whether conclusions drawn from long-term studies in the San Juan Islands, 5 Washington, about the relative importance of suspension feeding and kleptoparasitism can be 6 applied generally to T. cancellata across its biogeographic range, I expanded earlier studies to 7 various locales in Alaska and British Columbia. Kleptoparasitism is pervasive throughout T. 8 cancellata 's range, occurring with equal frequency throughout the areas studied. The average 9 density and size of worm hosts are relatively constant across this range. Snail and worm densities 10 are not significantly correlated at the larger scale of site (averaged over nearby sampling 11 locations clustered around a city), but are correlated at the smaller local scale (within a sampling 12 location). Larger worms do not support more snails. The abundance of uninfected worm hosts is 13 usually not limiting, except potentially in some sampling locations in southwest Alaska where 14 the use of a novel host (a sea cucumber) may be the result of low densities of uninfected worms.