The Epididymis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
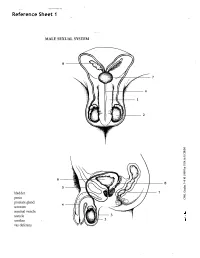
Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

MALE REPRODUCTIVE SYSTEM Male Reproduc�Ve System
Human Anatomy Unit 3 MALE REPRODUCTIVE SYSTEM Male Reproducve System • Gonads = testes – primary organ responsible for sperm producon – development/ maintenance of secondary sex characteriscs • Gametes = sperm Male Reproducve System Anatomy of the Testes • Tunica albuginea • Seminiferous tubules – highly coiled – sealed by the blood tess barrier – Site of sperm producon • located in tescular lobules Anatomy of the Testes Histology of the Testes • Intersal cells of Leydig – Intersal endocrinocytes – Located between seminiferous tubules – testosterone • Sertoli cells – Nursing cells or sustentacular cells – form the blood tess barrier – support sperm development Development of Sperm • Sperm formed by two processes – meiosis • Cell division resulng in cells with genecally varied cells with only one complete set of DNA (remember…our cells have two complete sets!) – spermatogenesis • morphological changes as sperm develop in tubule system • 64 days in humans – Can survive 3 days in female reproducve tract Development of Sperm The Long and Winding Road… • Seminiferous tubules • Rete tess • Epididymis • Vas deferens • Ejaculatory duct • Prostac urethra • Membranous urethra • Penile urethra The Epididymis • Sperm “swim school” • Comma shaped organ that arches over the posterior and lateral side of the tess • Stores spermatozoa unl ejaculaon or absorpon • Sperm stored for up to 2 weeks Vas Deferens • Extends from the epididymis • Passes posterior to the urinary bladder • Meets the spermac blood vessels to become the spermac cord • Enters -

Human Physiology/The Male Reproductive System 1 Human Physiology/The Male Reproductive System
Human Physiology/The male reproductive system 1 Human Physiology/The male reproductive system ← The endocrine system — Human Physiology — The female reproductive system → Homeostasis — Cells — Integumentary — Nervous — Senses — Muscular — Blood — Cardiovascular — Immune — Urinary — Respiratory — Gastrointestinal — Nutrition — Endocrine — Reproduction (male) — Reproduction (female) — Pregnancy — Genetics — Development — Answers Introduction In simple terms, reproduction is the process by which organisms create descendants. This miracle is a characteristic that all living things have in common and sets them apart from nonliving things. But even though the reproductive system is essential to keeping a species alive, it is not essential to keeping an individual alive. In human reproduction, two kinds of sex cells or gametes are involved. Sperm, the male gamete, and an egg or ovum, the female gamete must meet in the female reproductive system to create a new individual. For reproduction to occur, both the female and male reproductive systems are essential. While both the female and male reproductive systems are involved with producing, nourishing and transporting either the egg or sperm, they are different in shape and structure. The male has reproductive organs, or genitals, that are both inside and outside the pelvis, while the female has reproductive organs entirely within the pelvis. The male reproductive system consists of the testes and a series of ducts and glands. Sperm are produced in the testes and are transported through the reproductive ducts. These ducts include the epididymis, ductus deferens, ejaculatory duct and urethra. The reproductive glands produce secretions that become part of semen, the fluid that is ejaculated from the urethra. These glands include the seminal vesicles, prostate gland, and bulbourethral glands. -

Epididymo-Orchitis
Epididymo-orchitis In men over the age of 35 years the most Epididymo-orchitis Bladder common cause is a urine infection – with local Seminal spread of infection from the bladder. This may Epidiymo-orchitis – the basics vesicle Epididymo-orchitisIt is a condition- the basics affecting men characterised by also occur after surgical procedures such as pain and swelling inside the scrotum (ball bag) Prostate Rectum cystoscopy or catheterisation. Epididymo-orchitisand is duea tocondition an infection eitherthat in causesthe: pain and Urethra Occasionally it may also be due to a ‘gut’ swelling inside the scrotum (ball bag). epididymis – tube carrying the sperm from bacterial infection from insertive anal Te s t i s the testicle to the vas deferens and then the intercourse. It is due to an infectionurethra either or water in pipe the: (epididymitis) Rarely epididymo-orchitis may be caused by Penis • epididymistesticle – tube (orchitis) carrying the sperm from the other infections such as mumps or tuberculosis. testicle to theepididymis vas deferensand testicle (epididymo-orchitis)and then the Vas urethra or water pipe (epididymitis) deferens What would I notice if I had epididymo-orchitis? • In men under the age of 35 years it is usually A rapid onset of pain and swelling in one or testicle (orchitis) Epididymis caused by a sexually transmitted infection (STI) sometimes both of your testicles. • epididymisin theand water testicle pipe e.g. (epididymo chlamydia or gonorrhoea.-orchitis) Scrotal Te s t i s Some men may also notice a discharge from Skin Prompt medical assessment is needed to the tip of the water pipe and/or pain on passing In people undermake 35 sure theyou don’t infection have a twisted is testicleoften sexually urine. -

Scrotal Ultrasound
Scrotal Ultrasound Bruce R. Gilbert, MD, PhD Associate Clinical Professor of Urology & Reproductive Medicine Weill Cornell Medical College Director, Reproductive and Sexual Medicine Smith Institute For Urology North Shore LIJ Health System 1 Developmental Anatomy" Testis and Kidney Hindgut Allantois In the 3-week-old embryo the Primordial primordial germ cells in the wall of germ cells the yolk sac close to the attachment of the allantois migrate along the Heart wall of the hindgut and the dorsal Genital Ridge mesentery into the genital ridge. Yolk Sac Hindgut At 5-weeks the two excretory organs the pronephros and mesonephros systems regress Primordial Pronephric system leaving only the mesonephric duct. germ cells (regressing) Mesonephric The metanephros (adult kidney) system forms from the metanephric (regressing) diverticulum (ureteric bud) and metanephric mass of mesoderm. The ureteric bud develops as a dorsal bud of the mesonephric duct Cloaca near its insertion into the cloaca. Mesonephric Duct Mesonephric Duct Ureteric Bud Ureteric Bud Metanephric system Metanephric system 2 Developmental Anatomy" Wolffian and Mullerian DuctMesonephric Duct Under the influence of SRY, cells in the primitive sex cords differentiate into Sertoli cells forming the testis cords during week 7. Gonads Mesonephros It is at puberty that these testis cords (in Paramesonephric association with germ cells) undergo (Mullerian) Duct canalization into seminiferous tubules. Mesonephric (Wolffian) Duct At 7 weeks the indifferent embryo also has two parallel pairs of genital ducts: the Mesonephric (Wolffian) and the Paramesonephric (Mullerian) ducts. Bladder Bladder Mullerian By week 8 the developing fetal testis tubercle produces at least two hormones: Metanephros 1. A glycoprotein (MIS) produced by the Ureter Uterovaginal fetal Sertoli cells (in response to SRY) primordium Rectum which suppresses unilateral development of the Paramesonephric (Mullerian) duct 2. -

A C T a T H E R I O L O G I
ACTA THERIOLOGICA VOL. 18, 5: 107—118 BIALOWIE2A April, 1973 Alina KOWALSKA-DYRCZ The Structure of Internal Genital Organs in Zapodidae (Rodentia) |With Plates I & II] Investigations were carried out of histological structure of internal genital organs in four species of Zapodidae. The chief differences have been stated in the structure of gl. coagulantes, gl. bulbo-urethrales, colliculus seminalis, bulbus penis and farther part of the pars spongiosa urethrae in Sicistinae (Sicista) and in Zapodinae (Zapus, Napaeozapus). On the other hand, the structure of reproductive system in Zapodidae (especially in Sicista) is similar to that in Muroidea, this resemblance being particularly distinct in the structure and arrangement of the vesi- culae seminales, gl. coagulantes, gl. prostaticae and even in gl. bulbo- -urethrales. The similarities in the reproductive systems of Zapodidae, Dipodidae, Muroidea and Sciuridae have been discussed. I. INTRODUCTION Philogenetical relationships among rodents are not clear and disputable in many points. Therefore many authors are trying to improve the syste- matics of this group basing it on the structure of some internal organs. These should be the so-called conservative organs which are little de- pendent on the environmental selection. The reproductive system is undoubtly such a one. The attempts to base the systematics of rodents on the structure of the male genital organs have, among others, been undertaken by Vinogradov (1925), Mossman et al. (1932), Ara- t a (1964). The present paper discusses the structure of the male repro- ductive organs in some representatives of the family Zapodidae (superfa- mily Dipodoidea). [1071 108 A. Kowalska-Dyrcz II. MATERIAL AND METHODS The material used for the investigations consisted of genital organs taken from: five sexually mature males of Sicista betulina (Pallas, 1778), two males of Zapus hudsonicus (Z immerman n, 1780), two males of Zapus princeps J. -
The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

Non-Certified Epididymitis DST.Pdf
Clinical Prevention Services Provincial STI Services 655 West 12th Avenue Vancouver, BC V5Z 4R4 Tel : 604.707.5600 Fax: 604.707.5604 www.bccdc.ca BCCDC Non-certified Practice Decision Support Tool Epididymitis EPIDIDYMITIS Testicular torsion is a surgical emergency and requires immediate consultation. It can mimic epididymitis and must be considered in all people presenting with sudden onset, severe testicular pain. Males less than 20 years are more likely to be diagnosed with testicular torsion, but it can occur at any age. Viability of the testis can be compromised as soon as 6-12 hours after the onset of sudden and severe testicular pain. SCOPE RNs must consult with or refer all suspect cases of epididymitis to a physician (MD) or nurse practitioner (NP) for clinical evaluation and a client-specific order for empiric treatment. ETIOLOGY Epididymitis is inflammation of the epididymis, with bacterial and non-bacterial causes: Bacterial: Chlamydia trachomatis (CT) Neisseria gonorrhoeae (GC) coliforms (e.g., E.coli) Non-bacterial: urologic conditions trauma (e.g., surgery) autoimmune conditions, mumps and cancer (not as common) EPIDEMIOLOGY Risk Factors STI-related: condomless insertive anal sex recent CT/GC infection or UTI BCCDC Clinical Prevention Services Reproductive Health Decision Support Tool – Non-certified Practice 1 Epididymitis 2020 BCCDC Non-certified Practice Decision Support Tool Epididymitis Other considerations: recent urinary tract instrumentation or surgery obstructive anatomic abnormalities (e.g., benign prostatic -

Cranial Cavitry
Embryology Endo, Energy, and Repro 2017-2018 EMBRYOLOGY OF THE REPRODUCTIVE SYSTEM Janine Prange-Kiel, Ph.D. Office: L1.106, Phone: 83117 Email: [email protected] LEARNING OBJECTIVES: • Name the structures in kidney development that contribute to the development of the reproductive organs. • Predict how the presence or absence of the Y chromosome and the expression of the SRY gene would influence the development of the gonads. • Predict how the presence or absence of testosterone, dihydrotestosterone, and anit- Mullerian hormone would influence the development of the genital ducts and indifferent primordia of the external genitalia. I. Introduction In general, the function of the genital (reproductive) system in males and females is the formation, nurture, and transport of germ cells. In females, an additional function is to provide the proper milieu for the fetal development after conception. Like the urinary system, the genital system derives from intermediate mesoderm. The development of these two systems is tightly interwoven as structures that develop as parts of the urinary system gain function in the genital system. In the adult, the sexual organs differ between males and females. The early genital system, however, is similar in both sexes, and the sexual differentiation of this initially indifferent, bipotential system starts only in the seventh week of embryonic development. The details on how sexual differentiation is determined will be discussed below, but it is worth mentioning here that irregularities in this process result in disorders of sexual differentiation (DSDs). DSDs occur in approximately 1 in 4,500 live births and will be discussed in a separate lecture. -

The Development of the Intratesticular Excurrent Duct
Histology, Cytology and Embryology (HCE) Research Article ISSN: 2514-5940 The development of the intratesticular excurrent duct system of donkey (Equus asinus) from birth to maturity Abd-Elhafeez HH*, Moustafa MNK, Zayed AE and Sayed R Department of Anatomy and Histology, Assuit University, Egypt Abstract The testis of the donkey was completely descended into the scrotum around birth. It was covered by a thick tunica albuginea consisting of outer and inner fibrous layers in addition to middle vascular one. Discrete bundles of smooth muscle cells were demonstrated in the outer fibrous layer of the tunica albuginea. These cells give the tunica albuginea a contractile function, which may aid in sperm transport. The present study emphasized an irregular arrangement of the testicular lobules of the donkey during various stages of the postnatal life. Such arrangement does not allow some lobules to have direct contact with the mediastinum testis. The latter was located paraxially, toward the epididymal border and extended about two-thirds of the testicular length; it is thick at the head extremity and gradually faded out caudal wards. The connection between the seminiferous cords and the initial part of the straight tubules exhibited a sharp cut contact in neonates, but it demonstrated exfoliated supporting and germ cells, as well as crowded population of peritubular cells in suckling animals. In premature ones, the connection between the seminiferous tubules and straight testicular tubules was characterized by a peculiar structure appearance that attained more modification and development. This connection included two regions; terminal segment of the seminiferous tubule and a dilated initial part of the straight tubule.