Two Populations of Self-Maintaining Monocyte-Independent Macrophages Exist in Adult Epididymis and Testis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

MALE REPRODUCTIVE SYSTEM Male Reproduc�Ve System
Human Anatomy Unit 3 MALE REPRODUCTIVE SYSTEM Male Reproducve System • Gonads = testes – primary organ responsible for sperm producon – development/ maintenance of secondary sex characteriscs • Gametes = sperm Male Reproducve System Anatomy of the Testes • Tunica albuginea • Seminiferous tubules – highly coiled – sealed by the blood tess barrier – Site of sperm producon • located in tescular lobules Anatomy of the Testes Histology of the Testes • Intersal cells of Leydig – Intersal endocrinocytes – Located between seminiferous tubules – testosterone • Sertoli cells – Nursing cells or sustentacular cells – form the blood tess barrier – support sperm development Development of Sperm • Sperm formed by two processes – meiosis • Cell division resulng in cells with genecally varied cells with only one complete set of DNA (remember…our cells have two complete sets!) – spermatogenesis • morphological changes as sperm develop in tubule system • 64 days in humans – Can survive 3 days in female reproducve tract Development of Sperm The Long and Winding Road… • Seminiferous tubules • Rete tess • Epididymis • Vas deferens • Ejaculatory duct • Prostac urethra • Membranous urethra • Penile urethra The Epididymis • Sperm “swim school” • Comma shaped organ that arches over the posterior and lateral side of the tess • Stores spermatozoa unl ejaculaon or absorpon • Sperm stored for up to 2 weeks Vas Deferens • Extends from the epididymis • Passes posterior to the urinary bladder • Meets the spermac blood vessels to become the spermac cord • Enters -

Epididymo-Orchitis
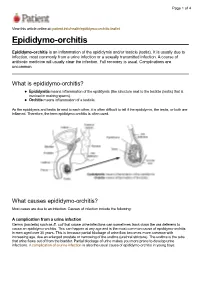
Epididymo-orchitis In men over the age of 35 years the most Epididymo-orchitis Bladder common cause is a urine infection – with local Seminal spread of infection from the bladder. This may Epidiymo-orchitis – the basics vesicle Epididymo-orchitisIt is a condition- the basics affecting men characterised by also occur after surgical procedures such as pain and swelling inside the scrotum (ball bag) Prostate Rectum cystoscopy or catheterisation. Epididymo-orchitisand is duea tocondition an infection eitherthat in causesthe: pain and Urethra Occasionally it may also be due to a ‘gut’ swelling inside the scrotum (ball bag). epididymis – tube carrying the sperm from bacterial infection from insertive anal Te s t i s the testicle to the vas deferens and then the intercourse. It is due to an infectionurethra either or water in pipe the: (epididymitis) Rarely epididymo-orchitis may be caused by Penis • epididymistesticle – tube (orchitis) carrying the sperm from the other infections such as mumps or tuberculosis. testicle to theepididymis vas deferensand testicle (epididymo-orchitis)and then the Vas urethra or water pipe (epididymitis) deferens What would I notice if I had epididymo-orchitis? • In men under the age of 35 years it is usually A rapid onset of pain and swelling in one or testicle (orchitis) Epididymis caused by a sexually transmitted infection (STI) sometimes both of your testicles. • epididymisin theand water testicle pipe e.g. (epididymo chlamydia or gonorrhoea.-orchitis) Scrotal Te s t i s Some men may also notice a discharge from Skin Prompt medical assessment is needed to the tip of the water pipe and/or pain on passing In people undermake 35 sure theyou don’t infection have a twisted is testicleoften sexually urine. -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

Non-Certified Epididymitis DST.Pdf
Clinical Prevention Services Provincial STI Services 655 West 12th Avenue Vancouver, BC V5Z 4R4 Tel : 604.707.5600 Fax: 604.707.5604 www.bccdc.ca BCCDC Non-certified Practice Decision Support Tool Epididymitis EPIDIDYMITIS Testicular torsion is a surgical emergency and requires immediate consultation. It can mimic epididymitis and must be considered in all people presenting with sudden onset, severe testicular pain. Males less than 20 years are more likely to be diagnosed with testicular torsion, but it can occur at any age. Viability of the testis can be compromised as soon as 6-12 hours after the onset of sudden and severe testicular pain. SCOPE RNs must consult with or refer all suspect cases of epididymitis to a physician (MD) or nurse practitioner (NP) for clinical evaluation and a client-specific order for empiric treatment. ETIOLOGY Epididymitis is inflammation of the epididymis, with bacterial and non-bacterial causes: Bacterial: Chlamydia trachomatis (CT) Neisseria gonorrhoeae (GC) coliforms (e.g., E.coli) Non-bacterial: urologic conditions trauma (e.g., surgery) autoimmune conditions, mumps and cancer (not as common) EPIDEMIOLOGY Risk Factors STI-related: condomless insertive anal sex recent CT/GC infection or UTI BCCDC Clinical Prevention Services Reproductive Health Decision Support Tool – Non-certified Practice 1 Epididymitis 2020 BCCDC Non-certified Practice Decision Support Tool Epididymitis Other considerations: recent urinary tract instrumentation or surgery obstructive anatomic abnormalities (e.g., benign prostatic -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

1 KEY WORDS: Testis, Epididymis, Torsion, Epididymitis, Ischemia
THE ACUTE SCROTUM KEY WORDS: Testis, epididymis, torsion, epididymitis, ischemia, tumor, infection, hernia LEARNING OBJECTIVES: At the end of medical school, the student should be able to: 1. Describe 6 conditions that may produce acute scrotal pain or swelling. 2. Distinguish, through the history, physical examination and laboratory testing, testicular torsion, torsion of testicular appendices, epididymitis, testicular tumor, scrotal trauma and hernia. 3. Appropriately order imaging studies to make the diagnosis of the acute scrotum. 4. Determine which acute scrotal conditions require emergent surgery and which may be handled less emergently or electively. INTRODUCTION: The “acute scrotum” may be viewed as the urologist’s equivalent to the general surgeon’s “acute abdomen.” Both conditions are guided by similar management principles: - The patient history and physical examination are key to the diagnosis and often guide decision making regarding whether or not surgical intervention is appropriate. - Imaging studies should complement, but not replace, sound clinical judgment. - When making a decision for conservative, non-surgical care, the provider must balance the potential morbidity of surgical exploration against the potential cost of missing a surgical diagnosis. - A small but real, negative exploration rate is acceptable to minimize the risk of missing a critical surgical diagnosis. DIFFERENTIAL DIAGNOSIS OF THE ACUTE SCROTUM A list of potential medical conditions that can present as acute pain or swelling of the scrotum are found -
Epididymo-Orchitis-Leaflet Epididymo-Orchitis
Page 1 of 4 View this article online at: patient.info/health/epididymo-orchitis-leaflet Epididymo-orchitis Epididymo-orchitis is an inflammation of the epididymis and/or testicle (testis). It is usually due to infection, most commonly from a urine infection or a sexually transmitted infection. A course of antibiotic medicine will usually clear the infection. Full recovery is usual. Complications are uncommon. What is epididymo-orchitis? Epididymitis means inflammation of the epididymis (the structure next to the testicle (testis) that is involved in making sperm). Orchitis means inflammation of a testicle. As the epididymis and testis lie next to each other, it is often difficult to tell if the epididymis, the testis, or both are inflamed. Therefore, the term epididymo-orchitis is often used. What causes epididymo-orchitis? Most cases are due to an infection. Causes of infection include the following: A complication from a urine infection Germs (bacteria) such as E. coli that cause urine infections can sometimes track down the vas deferens to cause an epididymo-orchitis. This can happen at any age and is the most common cause of epididymo-orchitis in men aged over 35 years. This is because partial blockage of urine flow becomes more common with increasing age, due an enlarged prostate or narrowing of the urethra (urethral stricture). The urethra is the tube that urine flows out of from the bladder. Partial blockage of urine makes you more prone to develop urine infections. A complication of a urine infection is also the usual cause of epididymo-orchitis in young boys. Page 2 of 4 Sexually transmitted infection A sexually transmitted infection is the most common cause of epididymo-orchitis in young men (but can occur in any sexually active man). -
Laboratory 8 - Urinary and Reproductive Systems
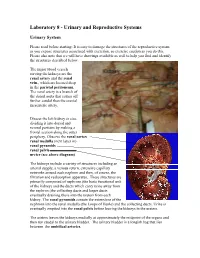
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Epididymis – Sperm Stasis
Epididymis – Sperm Stasis Figure Legend: Figure 1 Epididymis - Sperm Stasis. Ducts filled with sperm in a male B6C3F1 mouse from a chronic study. Figure 2 Epididymis - Sperm Stasis. Higher magnification of Figure 1 showing ducts filled with sperm in a male B6C3F1 mouse from a chronic study. Figure 3 Epididymis - Sperm Stasis. Sperm stasis is present on the left and ducts without sperm on the right in a male B6C3F1 mouse from a subchronic study. Figure 4 Epididymis - Sperm Stasis. Sperm stasis is present on the left and atrophic epididymal ducts on the right in a male F344/N rat from a chronic study. Comment: Sperm stasis is most commonly seen in the cauda epididymis following cessation of sperm production by the testis. Sperm become sequestered in the caudal ducts and are intensely eosinophilic (Figure 1, Figure 2, and Figure 3). The more proximal ducts are generally empty of sperm. Sperm stasis also occurs in the caput and efferent ducts as a result of obstructive lesions. In Figure 4, the 1 Epididymis – Sperm Stasis sequestered sperm are basophilic. Sperm stasis may progress to sperm granuloma if the epithelial lining is breached and sperm gain access to the interstitium. Recommendation: Sperm stasis should be recorded and graded and should be discussed in the pathology narrative if the incidence and/or severity appears to be related to chemical administration. Bilateral should be indicated when both epididymides are involved, with severity grade based on the more severely affected epididymis. Correlation with any spermatogenic disturbances should be noted in the pathology narrative to aid interpretation. -

Step-By-Step: Male Genital Examination Examination of Male Genitals and Secondary Sexual Characteristics
1 Clinical Summary Guide Step-by-Step: Male Genital Examination Examination of male genitals and secondary sexual characteristics. Testicular volume Testicular volume is assessed using an orchidometer; a sequential series of beads ranging from 1 mL to 35 mL (see Image 1). Testicular volume is measured using the following steps: 1. Conduct the examination in a warm environment, with the patient lying on his back 2. Gently isolate the testis and distinguish it from the epididymis. Then stretch the scrotal skin, without compressing the testis 3. Use your orchidometer to make a manual side-by-side comparison between the testis and beads (see image 2) 4. Identify the bead most similar in size to the testis, while making allowance not to include the scrotal skin. Normal testicular volume ranges Childhood Puberty Adulthood Image 1 – Orchidometer < 3 mL 4-14 mL 15-35 mL Why use an orchidometer? Clinical notes Testicular volume is important in the diagnosis of androgen • Asymmetry between testes is common (e.g. 15 mL versus deficiency, infertility and Klinefelter syndrome. 20 mL) and not medically significant • Asymmetry is sometimes more marked following unilateral testicular damage • Testes are roughly proportional to body size • Reduced testicular volume suggests impaired spermatogenesis • Small testes (<4 mL) from mid puberty are a consistent feature of Klinefelter syndrome Examination of secondary sexual characteristics Gynecomastia • Gynecomastia is the excessive and persistent development of benign glandular tissue evenly distributed in