SLAP Repair Utilizing the 3.0Mm Ti-Screw Suture Anchor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SLAP Repairs Versus Biceps Tenodesis in Athletes 15 Min Power Points
SLAP Repairs Versus Biceps Tenodesis in Athletes 15 min Power Points • Not all SLAP tears need surgery • Preservation of Native Anatomy GOAL • Not all labral repairs are equal • Kinetic chain MUST be addressed Power Points • Biceps DOES have a function • Tenodesis has consequences • Tenodesis relieves pain reliably BUT……long term effects uncertain ‘SLAPAHOLIC’ T. Romeo • One who fixes EVERY SLAP TEAR and anything that remotely looks like one! Not all SLAP Tears Need Surgery • SLAP tears way overdiagnosed • Beware of positive imaging study - negative exam • Slight labral separation may allow thrower to ‘get the slot’ MRI May OVERDIAGNOSE • Specificity ranges from 63% to 91% MRI and Anatomic Variants • Meniscoid labrum • Buford complex • Cord like MGHL • Age related attritional tear • ALL CAN LOOK LIKE SLAP TEARS ON MRI!! Meniscoid Labrum ‘Buford’ Complex Labral Tears are Part of the Aging Process! Pfahler et al JSES 2003 MANY LABRAL TEARS RESPOND TO REHAB!!!! Nonoperative Treatment of Superior Labrum Anterior Posterior Tears Improvements in Pain, Function, and Quality of Life Edwards et al • Approx. 50% of non operatively treated patients avoided surgery! Scapular strengthening, posterior capsular stretching Overtreat >>>> NIGHTMARE Make Sure History Consistent with SLAP “event” • Sudden loss of velocity (dead arm) • Large increase in pain • “mechanical symptoms” usually present • Rehab no longer effective Exam Hold Key!!! • Load Shift • Passive Distraction test • Mayo Shear • O’Brien’ Test (anterior) • Kim test • Relocation Test Mayo Shear -

THE MANAGEMENT of SLAP INJURIES in the OVERHEAD ATHLETE Michael G
THE MANAGEMENT of SLAP INJURIES in the OVERHEAD ATHLETE Michael G. Ciccotti, MD The Everett J. and Marian Gordon Professor of Orthopaedics Chief, Division of Sports Medicine Rothman Institute Thomas Jefferson University Head Team Physician, Philadelphia Phillies Faculty Disclosure Consultant – Stryker Endoscopy Consultant – Venture MD Research Support – Arthrex Board/Committee Membership – Orthopaedic Learning Center BOD MLB Medical Advisory Committee MLB Elbow Research Study Group AOSSM Fellowship Committee Rothman Institute of Orthopaedics at Thomas Jefferson University Shoulder Injury in the Athlete History Exam 27 y.o. RHD MLB 30 deg. less ABD, FF pitcher 25 deg. less INT ROT Chronic, progressive + Hawkin’s Test right shoulder pain + O’Brien’s & DLS while throwing Tests Worsened acutely + mild-mod supraspinatus Pain is deep and post weakness Unable to throw Rothman Institute of Orthopaedics at Thomas Jefferson University ShoulderDiagnosis: Injury SLAP in the Athlete Tear Rothman Institute of Orthopaedics at Thomas Jefferson University Superior Labrum is Important for the Overhead Athlete! Biceps attachment site Deepens glenoid Distributes contact pressure between humerus & glenoid Harryman, 1992 – Washer Effect Pagnani, 1995 Lee, 2005 Attachment site for glenohumeral Veeger, 2007 ligaments & capsule Lee, 2008 Kibler, 2011 Pressure sensor for proprioception Rothman Institute of Orthopaedics at Thomas Jefferson University Predictable Series of Events in Throwers Progressive Osseous changes Scapular and Cuff -

Rehab for Bicep Tendon Problems
Rehabilitation for Biceps Tendon Disorders and SLAP Lesions 117 capsulitis will suffer long-term ROM defi cits that may arthroscopic release (5); or MUA and arthroscopic last more than 10 years. Clarke et al. (1975) reported hydrodistension (20). During the long-term follow-up that 42% of patients continued to have motion loss (mean 52.3 months) 59% of patients reported hav- after 6 years of follow-up. Likewise, Schaffer et al. ing normal or near-normal shoulders, 35% reported (1992) reported that 50% of patients managed non- persistent mild/moderate symptoms, and 6% still had operatively remained symptomatic during their long- severe symptoms. Persistent symptoms were reported term follow-up, which occurred 2–11 years after their as mild in 94% of patients, with pain being the most initial visit (mean = 7 years). Of these patients, 60% common complaint. Only 6% of patients complained had a measurable restriction of shoulder motion. of severe pain and/or functional loss. Patients with External rotation was the most chronically restricted the most severe symptoms at condition onset had the movement, providing further evidence that the rotator worst long-term prognosis. In general, patients with interval and coracohumeral ligament are particularly comorbid factors, particularly diabetes, hyperthyroid- affected by adhesive capsulitis. Hand et al. (2008) ism, hypothyroidism, hypoadrenalism, Parkinson's tracked outcomes in 269 shoulders affected by pri- disease, cardiac disease, pulmonary disease, or cere- mary adhesive capsulitis who received no treatment brovascular accident, tend to have more severe and (95); physical therapy (55); steroid injection (139); longer lasting symptoms and tend to be more recalci- manipulation under anesthesia (MUA) (5); MUA and trant to treatment. -
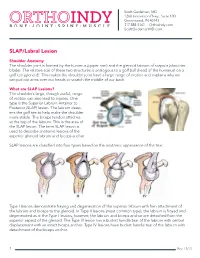
SLAP Lesions? the Shoulder’S Large, Though Useful, Range of Motion Can Also Lead to Injuries
Scott Gudeman, MD 1260 Innovation Pkwy., Suite 100 Greenwood, IN 46143 317.884.5161 OrthoIndy.com ScottGudemanMD.com SLAP/Labral Lesion Shoulder Anatomy The shoulder joint is formed by the humerus (upper arm) and the glenoid labrum of scapula (shoulder blade). The relative size of these two structures is analogous to a golf ball (head of the humerus) on a golf tee (glenoid). This makes the shoulder joint have a large range of motion and explains why we can put our arms over our heads or scratch the middle of our back. What are SLAP Lesions? The shoulder’s large, though useful, range of motion can also lead to injuries. One type is the Superior Labrum Anterior to Posterior (SLAP) lesion. The labrum deep- ens the golf tee to help make the shoulder more stable. The biceps tendon attaches at the top of the labrum. This is the area of the SLAP lesion. The term SLAP lesion is used to describe anatomic lesions of the superior glenoid labrum and biceps anchor. SLAP lesions are classified into four types based on the anatomic appearance of the tear. Type I lesions demonstrate fraying and degeneration of the superior labrum with firm attachment of the labrum and biceps to the glenoid. In Type II lesions (most common type), the labrum is frayed and degenerated as in the Type I lesions; however, the labrum and biceps anchor are detached from the superior aspect of the glenoid. The Type III lesion has a bucket handle tear of the labrum with central displacement with an intact biceps anchor. -

Prolotherapy: a Non-Invasive Approach to Lesions of the Glenoid Labrum; a Non-Controlled Questionnaire Based Study Ross A
Send Orders for Reprints to [email protected] The Open Rehabilitation Journal, 2013, 6, 69-76 69 Open Access Prolotherapy: A Non-Invasive Approach to Lesions of the Glenoid Labrum; A Non-Controlled Questionnaire Based Study Ross A. Hauser*, Erin Dolan and Amos Orlofsky Caring Medical and Rehabilitation Services, 715 Lake Street, Suite 600, Oak Park, IL 60301, USA Abstract: Lesions of the glenoid labrum are a common cause of shoulder instability and a frequent finding in patients with shoulder pain. Management of these patients typically involves an attempt to avoid surgery through conservative treatment. However, there is currently a dearth of conservative options that promote labral healing. Regenerative injection therapies, including prolotherapy, have shown promise in the treatment of several musculoskeletal disorders, but have not previously been applied to glenoid labral tear. Here we review several important aspects of these lesions and present an initial case series of 33 patients with labral tear that were treated in our clinic with intra-articular injections of hypertonic dextrose. Patient-reported assessments were collected by questionnaire at a mean follow-up time of 16 months. Treated patients reported highly significant improvements with respect to pain, stiffness, range of motion, crunching, exercise and need for medication. All 31 patients who reported pain at baseline experienced pain relief, and all 31 who reported exercise impairment at baseline reported improved exercise capability. Patients reported complete relief of 69% of recorded symptoms. One patient reported worsening of some symptoms. Prolotherapy for glenoid labral tear appears to be a safe procedure that merits further investigation. Keywords: Prolotherapy, glenoid labrum lesions, shoulder, hypertonic dextrose, musculosketetal repair, case report, regenerative injection therapy, glenoid labral tear. -

Biceps Tenodesis and Superior Labrum Anterior to Posterior (SLAP) Tears
5 Points on Biceps Tenodesis and Superior Labrum Anterior to Posterior (SLAP) Tears Mandeep S. Virk, MD, Annemarie K. Tilton, BS, and Brian J. Cole, MD, MBA njuries of the superior labrum–biceps complex (SLBC) presentation of SLAP tears, though localization and character- have been recognized as a cause of shoulder pain since ization of pain are variable and nonspecific.7 The mechanism Ithey were first described by Andrews and colleagues1 in of injury is helpful in acute presentation (traction injury; fall 1985. Superior labrum anterior to posterior (SLAP) tears are on outstretched, abducted arm), but an overhead athlete may relatively uncommon injuries of the shoulder, and their true present with no distinct mechanism other than chronic, re- incidence is difficult to establish. However, recently there petitive use of the shoulder.8-11 Numerous provocative physi- has been a significant increase in the reported incidence and cal examination tests have been used to assist in the diagnosis operative treatment of SLAP tears.2 SLAP tears can occur in of SLAP tear, yet there is no consensus regarding the ideal isolation, but they are commonly seen in association with physical examination test, with high sensitivity, specificity, other shoulder lesions, including rotator cuff tear, Bankart and accuracy.12-14 Magnetic resonance arthrography, the gold lesion, glenohumeral arthritis, acromioclavicular joint pa- standard imaging modality, is highly sensitive and specific thology, and subacromial impingement. (>95%) for diagnosing SLAP tears. Although SLAP tears are well described and classified,3-6 SLAP tear management is based on lesion type and se- our understanding of symptomatic SLAP tears and of their verity, age, functional demands, and presence of coexisting contribution to glenohumeral instability is limited. -

Shoulder Instability and Labral Tears
P.O. Box 660 85 Sierra Park Road Orthopedic Surgery & Sports Medicine Mammoth Lakes, CA 93546 SHOULDER: Instability • Dislocation • Labral Tears The shoulder is the most mobile joint in the body, but to have this amount of motion, it is also less stable and more likely to dislocate than other joints. The shoulder works like a ball and socket joint, but the bones are much more like a golf ball on a tee. The socket is called the glenoid, and the ball is the upper termination of the humerus (arm) bone. The labrum is a ring of specialized soft cartilage (similar to meniscus tissue in the knee) around the rim of the glenoid. It helps to make the glenoid socket slightly deeper and makes the shoulder much more stable. The rotator cuff muscles are also very important for shoulder stability—they actively pull and compress the humeral head against the socket. Rehab of the rotator cuff plays a large part in the recovering from injury or surgery. In a younger person’s shoulder, the labrum is often torn away from the bone when the shoulder dislocates (the ball comes all the way out of the socket) or the shoulder subluxes (the ball comes partially out of the socket). The labrum can also be damaged or torn with repetitive overhead use of the arm such as in throwing, tennis, and rock climbing. Damage to the labrum can be painful by itself, but most of the pain and disability comes from abnormal motion when the shoulder is used. The shoulder can also be unstable due to ligament or capsular laxity (looseness) and not specifically from a torn labrum. -
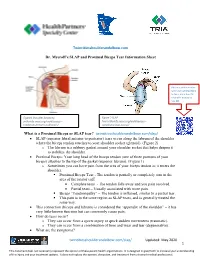
Dr. Myeroff's SLAP and Proximal Biceps Tear Information Sheet
Twincitiesshoulderandelbow.com Dr. Myeroff’s SLAP and Proximal Biceps Tear Information Sheet Please scan these codes with your camera phone to learn more from Dr. Myeroff’s website as you go! Figure1 Shoulder Anatomy Figure 2 SLAP orthoinfo.aaos.org/en/diseases -- Tearorthoinfo.aaos.org/en/diseases-- conditions/rotator-cuff-tears/ conditions/slap-tears// What is a Proximal Biceps or SLAP tear? twincitiesshoulderandelbow.com/slap/ • SLAP (superior labral anterior to posterior) tears occur along the labrum of the shoulder where the biceps tendon attaches to your shoulder socket (glenoid). (Figure 2) o The labrum is a rubbery gasket around your shoulder socket that helps deepen it to stabilize the shoulder. • Proximal Biceps: Your long head of the biceps tendon (one of three portions of your biceps) attaches to the top of the gasket (superior labrum). (Figure 1) o Sometimes you can have pain from the area of your biceps tendon as it enters the shoulder. ▪ Proximal Biceps Tear - The tendon is partially or completely torn in the area of the rotator cuff • Complete tears - The tendon falls away and you pain resolved. • Partial tears – Usually associated with more pain. ▪ Biceps “Tendonopathy” – The tendon is inflamed, similar to a partial tear. ▪ This pain is in the same region as SLAP tears, and is generally treated the same way. • This connection (biceps and labrum) is considered the “appendix of the shoulder” – it has very little-known function but can commonly cause pain. • How do tears occur? o They can occur from a sports injury or quick sudden movements (traumatic). o They can occur from a combination of time and wear and tear (degenerative). -

Arthroscopic SLAP Repair
Andrew Green, MD Associate Professor of Orthopaedic Surgery Chief of Shoulder and Elbow Surgery Warren Alpert Medical School, Brown University Arthroscopic Repair of Isolated Superior Labrum Anterior Posterior (SLAP) Tears Please follow the protocol along with the instructions listed on the patient’s referral This protocol was developed for patients who have had an arthroscopic repair of an isolated SLAP tear. The goal of this protocol is to advance range of motion and strength as directed while protecting the repair to ensure optimal healing. Please contact the physical therapy department at (401) 443-5000 if there are any questions. You may also refer to www.universityorthopedics.com and go to Dr. Green’s section to view video of the specific shoulder exercises: http://universityorthopedics.com/physicians/green/prepost.html Arthroscopic superior labral repair is typically performed in younger patients (less than 45 years old) when the tear is associated with destabilization of the attachment of the tendon of the long head of the biceps. Superior labral tears can be the result of traumatic injury or degenerative pathology. SLAP tears can be associated with rotator cuff tears as well as glenohumeral instability. Patients are discharged with a simple arm sling. Patients wear the sling for a total of 4 weeks after surgery. Active use of the shoulder is discouraged during this period. The patients may use their hand with the arm in the sling. The dressing is removed on the third day after surgery and the steristrips are left in place until the first post-operative office visit. After the dressing is removed the patients may shower quickly and gently pat the shoulder dry with a clean towel. -

Imaging of Sports Injury
Imaging of Sports Injuries Jonathan S. Luchs, MD Director, Musculoskeletal Imaging and Intervention Medical Director, Winthrop Radiology Associates, PC Program Director, Diagnostic Radiology Residency Winthrop-University Hospital Assistant Professor of Radiology SUNY Stony Brook School of Medicine Outline • Football Injuries • Baseball Injuries • Running Injuries • Martial Arts Injuries Football Injuries • Clipping • Turf Toe • Sports Hernia Clipping • “An illegal block in which a player hits an opponent from behind, typically at leg level.” Clipping O’Donohue’s Triad 1. ACL tear 2. MCL tear 3. Medial Meniscus Tear MCL Tear Cor PD Ax PD ACL Tear Sag T2 FS Sag PD MM Tear Sag Men Window Knee Dislocation Turf Toe • The name comes from the fact that this injury is especially common among athletes who play on artificial turf. • The hard surface of artificial turf, combined with running and jumping in football and soccer, make turf toe a frequent consequence of artificial turf play. Turf Toe • When a player sustains a turf toe injury they are tearing the 1st MTP joint capsule. • aka Plantar Plate Tear • Extremely painful. • Leads to instability and dislocation of the joint. • Leads to accelerated cartilage wear and arthritis of the 1st MTP joint. Plantar Plate Tear Abnormal High Signal Reflecting Capsule Tear Sag PD Sports Hernia • A sports hernia occurs when there is a weakening of the muscles or tendons of the lower abdominal wall. • This part of the abdomen is the same region where an inguinal hernia occurs. • In the case of a sports hernia, the problem is due to a weakening in the same abdominal wall muscles, but there is no palpable hernia or herniated tissue. -

SLAP Lesions Stable Shoulder
QUESTION | IS IT NECESSARY TO REPAIR A LARGE SLAP LESION IN A STABLE SHOULDER? IF THE SLAP LESION IS NOT REPAIRED AND A BICEPS TENOTOMY IS DONE (TO REDUCE THE STRESS ON THE LABRUM), WHAT ARE THE LONGER TERM FUNCTIONAL IMPLICATIONS? ANSWER | Andrews et al initially described tearing of the anterosuperior labrum from the glenoid. The original pathology was described in throwing athletes and occurring during the follow-through phase as traction was placed on the biceps tendon.1 Snyder et al later coined the phrase SLAP to represent lesions of the superior labrum from anterior to posterior.2 SLAP lesions have been commonly associated with trauma and overhead athletics. There have been ten SLAP lesions described, based on the location of torn labrum and biceps involvement. By far the most common SLAP lesion encountered is the Type II SLAP. Type II SLAP lesions are characterised by the combined detachment of the superior labrum and biceps tendon from the peripheral edge of the glenoid. They can often be found in isolation of other pathology and can be a primary source of shoulder pain. SLAP lesions are caused by either a traction or compression type injury to the labrum. Mechanisms include a fall on the outstretched arm, chronic acceleration/deceleration of the shoulder in throwing sports or labourers who constantly used their arms overhead. Patients with a stable glenohumeral joint and a SLAP lesion will often have a main complaint of shoulder pain. Pain usually occurs with use of the arm, especially with overhead or throwing activities. Clinical exam can raise suspicion of a SLAP tear by demonstrating positive special tests such as the Speed’s or O’Brien’s tests. -

Percutaneous SLAP Lesion Repair Technique Is an Effective Alternative to Portal of Wilmington
Percutaneous SLAP Lesion Repair Technique Is an Effective Alternative to Portal of Wilmington HOME OF: Welcome, Guest Log in | Mobile | RSS Entire Site Meetings Home Blogs News Wire Multimedia Classified Marketplace Education Lab SHOULDER/ELBOW SEE ALSO ORTHOPEDICS November 2010;33(11):803. Scapular Percutaneous SLAP Lesion Repair Technique Osteochondromas Treated With Surgical Is an Effective Alternative to Portal of Excision Meetings & Courses Wilmington ORTHOPEDICS November Featured Meetings 2010 by Gregory J. Galano, MD; Christopher S. Ahmad, MD; Louis Bigliani, MD; William Levine, MD Contact Pressure and Submit a Comment Print E-mail Glenohumeral Translation Following Subacromial EFORT Abstract Decompression: How Topics Athletes with superior labral tear from anterior to posterior (SLAP) lesions place large demands on their Much Is Enough? Arthritis rotator cuff and often have partial articular-sided rotator cuff tears as part of an internal impingement ORTHOPEDICS November Arthroscopy 2010 process. A percutaneous technique that facilitates SLAP repair may decrease the rotator cuff morbidity Biologics associated with establishment of the standard Wilmington portal. The current study reports the clinical Subscapularis Function Business of Orthopedics outcome of patients with SLAP lesions treated with a percutaneous repair technique. Twenty-two patients Following the Latarjet Coracoid Transfer for Foot and Ankle with SLAP lesions underwent percutaneous repair. Mean patient age was 26.9 years. Standard posterior viewing and anterior working portals were used. Anchor placement and suture passing were performed Recurrent Anterior Hand/Upper Extremity with a 3-mm percutaneous and transtendinous approach to the superior labrum. Knot tying was performed Shoulder Instability Hip via the standard anterior working portal.