Complement in Basic Processes of the Cell
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Complement Recognition Pathways in Renal Transplantation
BRIEF REVIEW www.jasn.org Complement Recognition Pathways in Renal Transplantation Christopher L. Nauser, Conrad A. Farrar, and Steven H. Sacks Medical Research Council Centre for Transplantation, Division of Transplantation Immunology and Mucosal Biology, King’s College London, National Health Service Guy’s and St. Thomas’ Trust, London, United Kingdom ABSTRACT The complement system, consisting of soluble and cell membrane–bound compo- weigh its effect on organ injury and nents of the innate immune system, has defined roles in the pathophysiology of renal rejection with dampening the antimi- allograft rejection. Notably, the unavoidable ischemia-reperfusion injury inherent to crobial functions of the complement sys- transplantation is mediated through the terminal complement activation products tem, such as opsonisation, cell lysis, and C5a and C5b-9. Furthermore, biologically active fragments C3a and C5a, produced recruitment of neutrophils and other in- during complement activation, can modulate both antigen presentation and T cell flammatory cells.7 It is our belief that priming, ultimately leading to allograft rejection. Earlier work identified renal tubule therapeutic strategies can be designed cell synthesis of C3, rather than hepatic synthesis of C3, as the primary source of C3 to specificallytargetthekeyinitiators driving these effects. Recent efforts have focused on identifying the local triggers of of complement activation at the relevant complement activation. Collectin-11, a soluble C-type lectin expressed in renal tis- location, such as complement-binding sue, has been implicated as an important trigger of complement activation in renal anti-HLA antibodies in the vascular tissue. In particular, collectin-11 has been shown to engage L-fucose at sites of compartment or the initiator(s) of local ischemic stress, activating the lectin complement pathway and directing the innate complement activation in the extravas- immune response to the distressed renal tubule. -

Genetic Susceptibility to Chronic Wasting Disease in Free-Ranging White-Tailed Deer: Complement Component C1q and Prnp Polymorphisms Julie A
Natural Resource Ecology and Management Natural Resource Ecology and Management Publications 12-2009 Genetic susceptibility to chronic wasting disease in free-ranging white-tailed deer: Complement component C1q and Prnp polymorphisms Julie A. Blanchong Iowa State University, [email protected] Dennis M. Heisey United States Geological Survey Kim T. Scribner Michigan State University Scot V. Libants Michigan State University Chad Johnson UFonilvloerwsit ythi of sW aiscondn asiddn - itMionadisoaln works at: http://lib.dr.iastate.edu/nrem_pubs Part of the Animal Diseases Commons, Genetics Commons, Natural Resources Management See next page for additional authors and Policy Commons, Veterinary Infectious Diseases Commons, and the Zoology Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ nrem_pubs/84. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Natural Resource Ecology and Management at Iowa State University Digital Repository. It has been accepted for inclusion in Natural Resource Ecology and Management Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Genetic susceptibility to chronic wasting disease in free-ranging white- tailed deer: Complement component C1q and Prnp polymorphisms Abstract The eg netic basis of susceptibility to chronic wasting disease (CWD) in free-ranging cervids is of great interest. Association studies of disease susceptibility in free-ranging populations, however, face considerable challenges including: the need for large sample sizes when disease is rare, animals of unknown pedigree create a risk of spurious results due to population admixture, and the inability to control disease exposure or dose. -

Neutrophil Chemoattractant Receptors in Health and Disease: Double-Edged Swords
Cellular & Molecular Immunology www.nature.com/cmi REVIEW ARTICLE Neutrophil chemoattractant receptors in health and disease: double-edged swords Mieke Metzemaekers1, Mieke Gouwy1 and Paul Proost 1 Neutrophils are frontline cells of the innate immune system. These effector leukocytes are equipped with intriguing antimicrobial machinery and consequently display high cytotoxic potential. Accurate neutrophil recruitment is essential to combat microbes and to restore homeostasis, for inflammation modulation and resolution, wound healing and tissue repair. After fulfilling the appropriate effector functions, however, dampening neutrophil activation and infiltration is crucial to prevent damage to the host. In humans, chemoattractant molecules can be categorized into four biochemical families, i.e., chemotactic lipids, formyl peptides, complement anaphylatoxins and chemokines. They are critically involved in the tight regulation of neutrophil bone marrow storage and egress and in spatial and temporal neutrophil trafficking between organs. Chemoattractants function by activating dedicated heptahelical G protein-coupled receptors (GPCRs). In addition, emerging evidence suggests an important role for atypical chemoattractant receptors (ACKRs) that do not couple to G proteins in fine-tuning neutrophil migratory and functional responses. The expression levels of chemoattractant receptors are dependent on the level of neutrophil maturation and state of activation, with a pivotal modulatory role for the (inflammatory) environment. Here, we provide an overview -
![Cd1a [O10] Concentrated and Prediluted Monoclonal Antibody 901-3158-061719](https://docslib.b-cdn.net/cover/1446/cd1a-o10-concentrated-and-prediluted-monoclonal-antibody-901-3158-061719-341446.webp)
Cd1a [O10] Concentrated and Prediluted Monoclonal Antibody 901-3158-061719
CD1a [O10] Concentrated and Prediluted Monoclonal Antibody 901-3158-061719 Catalog Number: ACI 3158 A, B API 3158 AA VLTM 3158 G20 Description: 0.1, 0.5 mL conc. 6.0 mL, RTU 20 mL, RTU Dilution: 1:100 Ready-to-use Ready-to-use Diluent: Van Gogh Yellow N/A N/A Intended Use: Protocol Recommendations (VALENT® Automated Slide For In Vitro Diagnostic Use Staining Platform) Cont’d: CD1a [O10] is a mouse monoclonal antibody that is intended for Protein Block (Optional): Incubate for 10-20 minutes at RT with Val laboratory use in the qualitative identification of CD1a protein by Background Block. immunohistochemistry (IHC) in formalin-fixed paraffin-embedded Primary Antibody: Incubate for 30 minutes. (FFPE) human tissues. The clinical interpretation of any staining or its Secondary: Incubate for 10 minutes with Val Mouse Secondary. absence should be complemented by morphological studies using proper Linker: Incubate for 10 minutes with Val Universal Linker. controls and should be evaluated within the context of the patient’s Polymer: Incubate for 10 minutes with Val Universal Polymer. clinical history and other diagnostic tests by a qualified pathologist. Chromogen: Incubate for 5 minutes with Val DAB. Summary and Explanation: Counterstain: Counterstain for 5 minutes with Val Hematoxylin. CD1a is a protein of 43 to 49 kDa and is expressed on dendritic cells and cortical thymocytes (1,2). CD1a [O10] staining has been shown to be Protocol Recommendations (intelliPATH FLX® and manual use): useful in the differentiation of Langerhans cells from interdigitating cells. Peroxide Block: Block for 5 minutes with Peroxidazed 1. It has also proved useful for phenotyping Langerhans cell histiocytosis Pretreatment: Perform heat retrieval using Diva or Reveal Decloaker. -

An Anticomplement Agent That Homes to the Damaged Brain and Promotes Recovery After Traumatic Brain Injury in Mice
An anticomplement agent that homes to the damaged brain and promotes recovery after traumatic brain injury in mice Marieta M. Rusevaa,1,2, Valeria Ramagliab,1, B. Paul Morgana, and Claire L. Harrisa,3 aInstitute of Infection and Immunity, School of Medicine, Cardiff University, Cardiff CF14 4XN, United Kingdom; and bDepartment of Genome Analysis, Academic Medical Center, Amsterdam 1105 AZ, The Netherlands Edited by Douglas T. Fearon, Cornell University, Cambridge, United Kingdom, and approved September 29, 2015 (received for review July 15, 2015) Activation of complement is a key determinant of neuropathology to rapidly and specifically inhibit MAC at sites of complement and disability after traumatic brain injury (TBI), and inhibition is activation, and test its therapeutic potential in experimental TBI. neuroprotective. However, systemic complement is essential to The construct, termed CD59-2a-CRIg, comprises CD59a linked fight infections, a critical complication of TBI. We describe a to CRIg via the murine IgG2a hinge. CD59a prevents assembly targeted complement inhibitor, comprising complement receptor of MAC in cell membranes (16), whereas CRIg binds C3b/iC3b of the Ig superfamily (CRIg) fused with complement regulator CD59a, deposited at sites of complement activation (17). The IgG2a designed to inhibit membrane attack complex (MAC) assembly at hinge promotes dimerization to increase ligand avidity. CD59- sites of C3b/iC3b deposition. CRIg and CD59a were linked via the 2a-CRIg protected in the TBI model, demonstrating that site- IgG2a hinge, yielding CD59-2a-CRIg dimer with increased iC3b/C3b targeted anti-MAC therapeutics may be effective in prevention binding avidity and MAC inhibitory activity. CD59-2a-CRIg inhibited of secondary neuropathology and improve neurologic recovery MAC formation and prevented complement-mediated lysis in vitro. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -
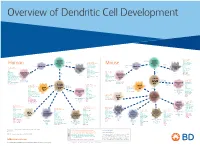
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit) -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

In Vitro Selection for Adhesion of Plasmodium Falciparum-Infected Erythrocytes to ABO Antigens Does Not Affect Pfemp1 and RIFIN
www.nature.com/scientificreports OPEN In vitro selection for adhesion of Plasmodium falciparum‑infected erythrocytes to ABO antigens does not afect PfEMP1 and RIFIN expression William van der Puije1,2, Christian W. Wang 4, Srinidhi Sudharson 2, Casper Hempel 2, Rebecca W. Olsen 4, Nanna Dalgaard 4, Michael F. Ofori 1, Lars Hviid 3,4, Jørgen A. L. Kurtzhals 2,4 & Trine Staalsoe 2,4* Plasmodium falciparum causes the most severe form of malaria in humans. The adhesion of the infected erythrocytes (IEs) to endothelial receptors (sequestration) and to uninfected erythrocytes (rosetting) are considered major elements in the pathogenesis of the disease. Both sequestration and rosetting appear to involve particular members of several IE variant surface antigens (VSAs) as ligands, interacting with multiple vascular host receptors, including the ABO blood group antigens. In this study, we subjected genetically distinct P. falciparum parasites to in vitro selection for increased IE adhesion to ABO antigens in the absence of potentially confounding receptors. The selection resulted in IEs that adhered stronger to pure ABO antigens, to erythrocytes, and to various human cell lines than their unselected counterparts. However, selection did not result in marked qualitative changes in transcript levels of the genes encoding the best-described VSA families, PfEMP1 and RIFIN. Rather, overall transcription of both gene families tended to decline following selection. Furthermore, selection-induced increases in the adhesion to ABO occurred in the absence of marked changes in immune IgG recognition of IE surface antigens, generally assumed to target mainly VSAs. Our study sheds new light on our understanding of the processes and molecules involved in IE sequestration and rosetting. -

The Complement System: a Powerful Modulator and Effector of Astrocyte Function in the Healthy and Diseased Central Nervous System
cells Review The Complement System: A Powerful Modulator and Effector of Astrocyte Function in the Healthy and Diseased Central Nervous System Marcela Pekna 1,3,4,* and Milos Pekny 2,3,4 1 Laboratory of Regenerative Neuroimmunology, Center for Brain Repair, Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 40530 Gothenburg, Sweden 2 Laboratory of Astrocyte Biology and CNS Regeneration, Center for Brain Repair, Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 40530 Gothenburg, Sweden; [email protected] 3 Florey Institute of Neuroscience and Mental Health, Parkville, Melbourne 3010, Australia 4 School of Medicine and Public Health, University of Newcastle, Newcastle 2308, Australia * Correspondence: [email protected]; Tel.: +46-31-786-3581 Abstract: The complement system, an effector arm of the innate immune system that plays a critical role in tissue inflammation, the elimination of pathogens and the clearance of dead cells and cell debris, has emerged as a regulator of many processes in the central nervous system, including neural cell genesis and migration, control of synapse number and function, and modulation of glial cell responses. Complement dysfunction has also been put forward as a major contributor to neurological disease. Astrocytes are neuroectoderm-derived glial cells that maintain water and ionic homeostasis, and control cerebral blood flow and multiple aspects of neuronal functioning. By virtue of their Citation: Pekna, M.; Pekny, M. The Complement System: A Powerful expression of soluble as well as membrane-bound complement proteins and receptors, astrocytes are Modulator and Effector of Astrocyte able to both send and receive complement-related signals. -

Gene List HTG Edgeseq Immuno-Oncology Assay
Gene List HTG EdgeSeq Immuno-Oncology Assay Adhesion ADGRE5 CLEC4A CLEC7A IBSP ICAM4 ITGA5 ITGB1 L1CAM MBL2 SELE ALCAM CLEC4C DST ICAM1 ITGA1 ITGA6 ITGB2 LGALS1 MUC1 SVIL CDH1 CLEC5A EPCAM ICAM2 ITGA2 ITGAL ITGB3 LGALS3 NCAM1 THBS1 CDH5 CLEC6A FN1 ICAM3 ITGA4 ITGAM ITGB4 LGALS9 PVR THY1 Apoptosis APAF1 BCL2 BID CARD11 CASP10 CASP8 FADD NOD1 SSX1 TP53 TRAF3 BCL10 BCL2L1 BIRC5 CASP1 CASP3 DDX58 NLRP3 NOD2 TIMP1 TRAF2 TRAF6 B-Cell Function BLNK BTLA CD22 CD79A FAS FCER2 IKBKG PAX5 SLAMF1 SLAMF7 SPN BTK CD19 CD24 EBF4 FASLG IKBKB MS4A1 RAG1 SLAMF6 SPI1 Cell Cycle ABL1 ATF1 ATM BATF CCND1 CDK1 CDKN1B NCL RELA SSX1 TBX21 TP53 ABL2 ATF2 AXL BAX CCND3 CDKN1A EGR1 REL RELB TBK1 TIMP1 TTK Cell Signaling ADORA2A DUSP4 HES1 IGF2R LYN MAPK1 MUC1 NOTCH1 RIPK2 SMAD3 STAT5B AKT3 DUSP6 HES5 IKZF1 MAF MAPK11 MYC PIK3CD RNF4 SOCS1 STAT6 BCL6 ELK1 HEY1 IKZF2 MAP2K1 MAPK14 NFATC1 PIK3CG RORC SOCS3 SYK CEBPB EP300 HEY2 IKZF3 MAP2K2 MAPK3 NFATC3 POU2F2 RUNX1 SPINK5 TAL1 CIITA ETS1 HEYL JAK1 MAP2K4 MAPK8 NFATC4 PRKCD RUNX3 STAT1 TCF7 CREB1 FLT3 HMGB1 JAK2 MAP2K7 MAPKAPK2 NFKB1 PRKCE S100B STAT2 TYK2 CREB5 FOS HRAS JAK3 MAP3K1 MEF2C NFKB2 PTEN SEMA4D STAT3 CREBBP GATA3 IGF1R KIT MAP3K5 MTDH NFKBIA PYCARD SMAD2 STAT4 Chemokine CCL1 CCL16 CCL20 CCL25 CCL4 CCR2 CCR7 CX3CL1 CXCL12 CXCL3 CXCR1 CXCR6 CCL11 CCL17 CCL21 CCL26 CCL5 CCR3 CCR9 CX3CR1 CXCL13 CXCL5 CXCR2 MST1R CCL13 CCL18 CCL22 CCL27 CCL7 CCR4 CCRL2 CXCL1 CXCL14 CXCL6 CXCR3 PPBP CCL14 CCL19 CCL23 CCL28 CCL8 CCR5 CKLF CXCL10 CXCL16 CXCL8 CXCR4 XCL2 CCL15 CCL2 CCL24 CCL3 CCR1 CCR6 CMKLR1 CXCL11 CXCL2 CXCL9 CXCR5