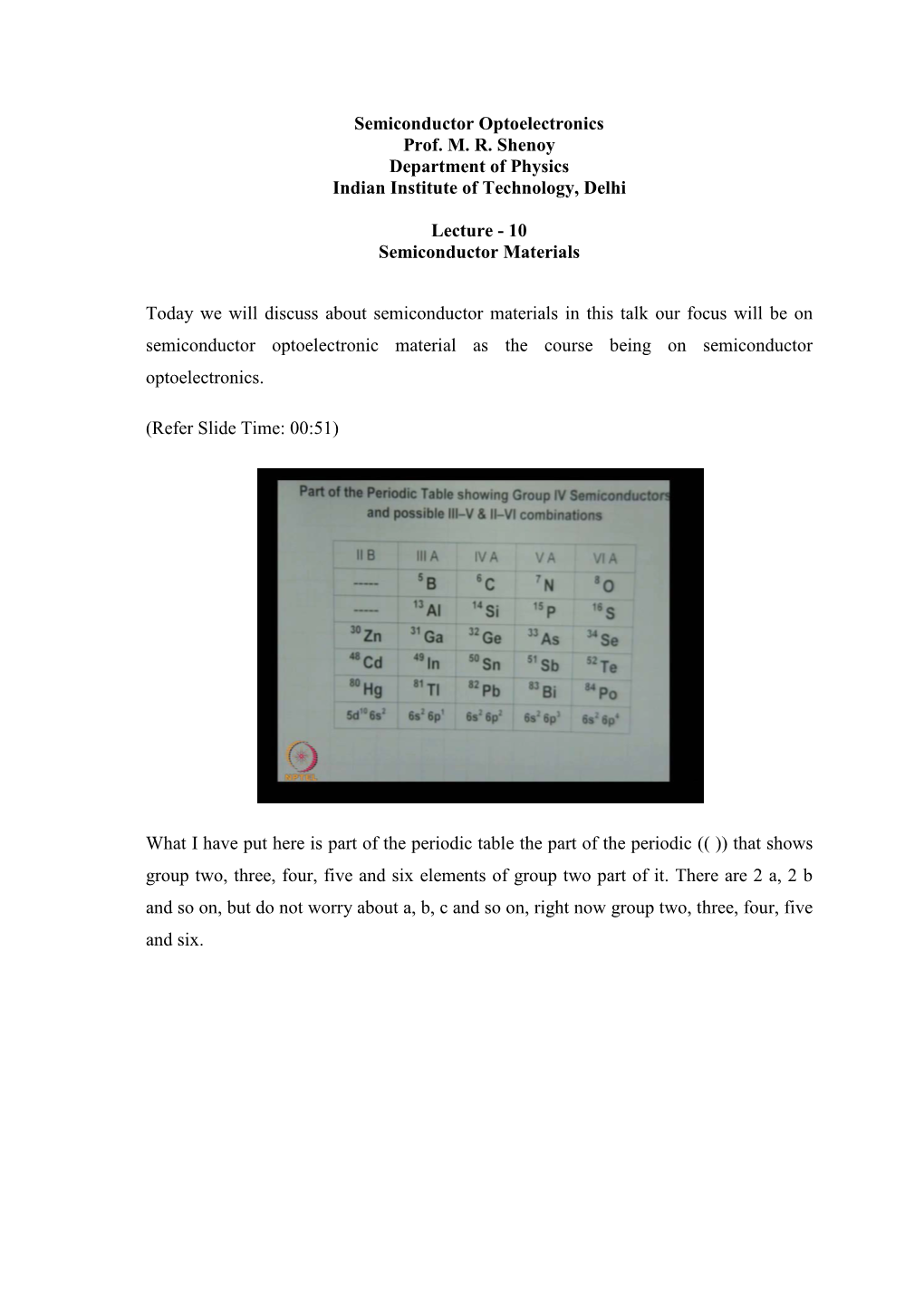
Semiconductor Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Guide to Export Controls
Foreign Affairs, Trade and Affaires étrangères, Commerce et Development Canada Développment Canada A Guide To CANADA’S EXPORT CONTROLS December 2012 Introduction The issuance of export permits is administered by the Export Controls Division (TIE) of Foreign Affairs, Trade and Development Canada (DFATD). TIE provides assistance to exporters in determining if export permits are required. It also publishes brochures and Notices to Exporters that are freely available on request and on our website www.exportcontrols.gc.ca. How to contact us: Export Controls Division (TIE) Foreign Affairs, Trade and Development Canada 111 Sussex Drive Ottawa, Ontario K1A 0G2 Telephone: (613) 996-2387 Facsimile: (613) 996-9933 Email: [email protected] For information on how to apply for an export permit and additional information on export controls please refer to our website. To enquire on the status of an export permit application: Recognized EXCOL users can check the status of an export permit application on-line. Non-recognized users can call (613) 996-2387 or email [email protected] and quote your export permit application identification (ref ID) number. Export Controls Division website: www.exportcontrols.gc.ca This Guide, at time of publication, encompasses the list of items enumerated on the Export Control List (ECL) that are controlled for export in accordance with Canadian foreign policy, including Canada’s participation in multilateral export control regimes and bilateral agreements. Unless otherwise specified, the export controls contained in this Guide apply to all destinations except the United States. Canada’s Export Control List can be found at the Department of Justice website at http://canada.justice.gc.ca/. -

Communications
COMMUNICATIONS dihydroxynaphthalene or 3,4-dihydroxybenzoate (extinction of blue qualify for Zintl phases when a broader definition is used. The l fluorescence at em 440 nm upon oxidation to the quinone). Nor- latter includes compounds of transition metals with filled or adrenaline reacts similarly to adrenaline down to pH 5. Adreno- empty d shells, that is the late transition metals of the Ni, Cu, chrome (2) is unstable and polymerizes to brown and finally insoluble [2] black products upon prolonged standing in solution. and Zn groups, and the early transition elements of the Ti, V, [14] a) M. Simmel, M. Turunen, J. Piironen, T. Vaara, VTT Symp. 1991, 122, and Cr groups at maximum formal oxidation states.[3] There 145 ± 161; b) R. J. Wodzinski, A. H. J. Ullah, Adv. Appl. Microbiol. are only two Zintl compounds containing a transition metal 1996, 42, 263 ± 302. with partially filled d shell, both based on manganese, [15] B.-L. Liu, A. Rafiq, Y.-M. Tzeng, A. Rob, Enzyme Microbiol. Technol. 1998, 22, 415 ± 424. (AE)14MnPn11 (AE alkaline-earth metal, Pn pnictogen) [4] [16] A. H. J. Ullah, D. M. Gibson, Prep. Biochem. 1987, 17, 63 ± 91. and Sr21Mn4Sb18 . Perhaps only they should be called true [17] Several kits for the titration of inorganic phosphate based on the ™transition metal Zintl phases∫ although the name is contra- reaction with ammonium molybdate are available commercially. dictory in itself. All but two of the d0 compounds contain Methyl-umbelliferyl phosphate and 4-nitrophenyl phosphate are isolated tetrahedra [MPn ]nÀ (M Nb, Ta, W, Ti).[5] The two general fluorogenic and chromogenic substrates for phosphatases 4 but do not enable phytases to be distinguished from other phospha- exceptions are Na5HfAs3 with dimers of edge-sharing tetra- 10À tases, in contrast to assays based on phytic acid. -

The Reaction of Pyridine Witb Sulfur Dioxide In
The Reaction of Pyridine witb Sulfur Dioxide in Benzoyl Chloride (55 letters) J THE REACTION OF PYRIDINE WITH SULFUR DIOXIDE IN BENZOYL CHLORIDE by James R. Watson A Thesis submitted to the Faculty of Graduate Studies and Research in partial fulfilment of the requirements for the degree of Master of Science Department of Chemistry McGill University Montreal, Canada August, 1965 • - i - ACKNOWLEDGMENTS I wish to thank Dr. J. T. Edward for his helpful guidance during my stay at McGill University and in the preparation of this manuscript • • TABLE OF CONTENTS ACKNOWLEDGMENTS. • . • • . • . • i LIST OF FIGURES........................................ ii LIST OF TABLES.. • • . • • . • . • . • 1 v INTRODUCTION•••••••••••••••·•·••••••••••••••••••••••••• 1 A. Reactions of Acylpyridinium Halides.............. 2 B. Partial Reduction to Dihydro and Tetrahydro Pyridine.................................. 6 C. Ring Opening Reactions........................... 8 EXPERIMENTAL. • • • • . • . • • • . • . • • • • • . • • . • • • . • • • . • 11 A. Reagents............... .. ... .. 11 B. Reaction of Pyridine with Sulfur Dioxide in Benzoyl Chloride to form Compound A.............. 11 C. Measurement of the Dissociation Constant of Compound A....................................... 15 D. Attempted Reaction of Pyridine, Benzoyl Chloride and Sodium Sulfite............................... 18 E. Reaction of 2-Picoline with Sulfur Dioxide in Benzoyl Chloride ta form Compound B.............. 18 F. Reaction of 2,4-Lutidine with Sulfur Dioxide in Benzoyl Chloride to form -

Modelling and Optimising Gaas/Al (X) Ga (1-X) As Multiple Quantum Well
University of London Imperial College of Science, Technology and Medicine Department of Physics Modelling and Optimising GaAs/AlxGa1 xAs − Multiple Quantum Well Solar Cells James P. Connolly arXiv:1006.1053v1 [cond-mat.mes-hall] 5 Jun 2010 Submitted in part fulfilment of the requirements for the degree of Doctor of Philosophy in Science of the University of London and the Diploma of Imperial College, January 1997 2 Abstract The quantum well solar cell (QWSC) is a p - i - n solar cell with quantum wells in the intrinsic region. Previous work has shown that QWSCs have a greater open circuit voltage (Voc) than would be provided by a cell with the quantum well effective bandgap. This suggests that the fundamental efficiency limits of QWSCs are greater than those of single bandgap solar cells. The following work investigates QWSCs in the GaAs/AlxGa1−xAs materials system. The design and optimisation of a QWSC in this system requires studies of the voltage and current dependencies on the aluminium fraction. QWSCs with different aluminium fractions have been studied and show an increasing Voc with increasing barrier aluminium composition. The QE however decreases with increasing aluminium composition. We de- velop a model of the QE to test novel QWSC designs with a view to minimising this problem. This work concentrates on two design changes. The first deals with com- positionally graded structures in which the bandgap varies with position. This bandgap variation introduces an quasi electric field which can be used to increase minority carrier collection in the low efficiency p and n layers. This technique also increases the light flux reaching the highly efficient depletion regions. -

Determination of Band Structure of Gallium-Arsenide and Aluminium-Arsenide Using Density Functional Theory
Computational Chemistry, 2016, 4, 73-82 Published Online July 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.43007 Determination of Band Structure of Gallium-Arsenide and Aluminium-Arsenide Using Density Functional Theory J. A. Owolabi1, M. Y. Onimisi1, S. G. Abdu2, G. O. Olowomofe1 1Department of Physics, Nigerian Defence Academy, Kaduna, Nigeria 2Department of Physics, Kaduna State University, Kaduna, Nigeria Received 18 April 2016; accepted 2 July 2016; published 5 July 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract This research paper is on Density Functional Theory (DFT) within Local Density Approximation. The calculation was performed using Fritz Haber Institute Ab-initio Molecular Simulations (FHI- AIMS) code based on numerical atomic-centered orbital basis sets. The electronic band struc- ture, total density of state (DOS) and band gap energy were calculated for Gallium-Arsenide and Aluminium-Arsenide in diamond structures. The result of minimum total energy and computa- tional time obtained from the experimental lattice constant 5.63 A for both Gallium Arsenide and Aluminium Arsenide is −114,915.7903 eV and 64.989 s, respectively. The electronic band structure analysis shows that Aluminium-Arsenide is an indirect band gap semiconductor while Gallium-Arsenide is a direct band gap semiconductor. The energy gap results obtained for GaAs is 0.37 eV and AlAs is 1.42 eV. The band gap in GaAs observed is very small when compared to AlAs. -

Quaternary Ammonium Compositions and Their Uses
Europaisches Patentamt (19) European Patent Office Office europeen des brevets (11) EP 0 726 246 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) |nt. CI.6: C07C 21 1/63, C01 B 33/44, 14.08.1996 Bulletin 1996/33 C1 p 1/62j Q21 C 5/02, (21) Application number: 96101900.7 A61 K 7/50 //C09D7/12 (22) Date of filing: 09.02.1996 (84) Designated Contracting States: • Campbell, Barbara DE DK ES FR GB IT NL Bristol, PA 1 9007 (US) • Chiavoni, Araxi (30) Priority: 10.02.1995 US 385295 Trenton, N J 0861 0 (US) • Magauran, Edward (71 ) Applicant: RHEOX INTERNATIONAL, INC. Westhampton, NJ 08060 (US) Hightstown, New Jersey 08520 (US) (74) Representative: Strehl Schubel-Hopf Groening & (72) Inventors: Partner • Cody, Charles, Dr. Maximilianstrasse 54 Robbinsville, NJ 08691 (US) 80533 Munchen (DE) (54) Quaternary ammonium compositions and their uses (57) Quaternary ammonium compositions are described which are made using diluents including soya bean oil, caster oil, mineral oils, isoparaffin/naphthenic and coconut oil. Such diluents remain as diluents in the final product and generally have a vapor pressure of 1mm of Hg or less at 25°C, and are liquid at ambient temperature. The quaternary/ammonium diluent com- positions have low volatile organic compound emission rates and high flash points, and can be tailored to partic- ular applications. Such applications include use a fabric softeners, cosmetics ingredients, deinking additives, surfactants, and reaction materials in the manufacture of organoclays. < CO CM CO CM o Q_ LU Printed by Rank Xerox (UK) Business Services 2.13.0/3.4 EP 0 726 246 A1 Description BACKGROUND OF THE INVENTION 5 1 . -

Khatiwada-Dissertation-2019
©Copyright by Devendra Khatiwada 2019 All Rights Reserved Fabrication and Optimization of Single-Junction GaAs Thin Film Solar Cells on Epi-ready Flexible Metal Tapes for Low-cost Photovoltaics A Dissertation Presented to the Faculty of the Department of Mechanical Engineering University of Houston In Partial Fulfillment for the Requirements of the Degree Doctor of Philosophy in Materials Science and Engineering by Devendra Khatiwada August 2019 Fabrication and Optimization of Single-Junction GaAs Thin Film Solar Cells on Epi-ready Flexible Metal Tapes for Low-cost Photovoltaics Devendra Khatiwada Approved: Chair of the Committee Venkat Selvamanickam, Professor, Mechanical Engineering Committee Members: Haleh Ardebili, Associate Professor, Mechanical Engineering James K.Meen, Research Associate Professor, Chemistry Jae -Hyun Ryou, Associate Professor, Mechanical Engineering M e c Alamgir Karim, Associate Professor, h Material Science and Engineering a n i c Suresh K.Khator, Associate Dean, Alamgir Karim, Professor and a Cullen College of Engineering Director, Material Science and l Engineering E n g ii i n e e r Acknowledgements Firstly I would like to acknowledge my thesis advisor professor Venkat Selvamanickam for his persistent help, guidance and offering me opportunities to involve in various interesting project. The last four years has been an outstanding experience to work with him. His patience in research, scientific knowledge, hard work and technical skills always inspired me to continuously work in different projects. I am very lucky to have him as my advisor. It was a great pleasure to work with the excellent members in our research group. Special thanks to Dr.Pavel Dutta, Dr.Monika Rathi, Sicong Son, Carlos Favela, Sahil Sharma, Sara Pouladi for their great help during my research work. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Available Online Journal of Scientific And
Available online www.jsaer.com Journal of Scientific and Engineering Research, 2016, 3(5):281-284 ISSN: 2394-2630 CODEN(USA): JSERBR Research Article Study of toxicity of arsenic during the synthesis of new class of iron-based oxyarsenide superconductors Intikhab A Ansari, Syed S Ghani*, Iqbal Hussain General Studies Department, Jubail Industrial College, P. O. Box - 10099, Jubail industrial City-31961, Saudi Arabia Abstract The toxicity of arsenic and its severe effect on human-beings are reported in this study. The different exposure limits of various materials are described during the synthesis of iron-based superconductor as a time- weighted average (TWA) over an 8-hours workday. This report reveals that the element arsenic is found to be highly toxic in case of inhalation or ingestion by the employers amongst the starting materials during the synthesis of oxyarsenide superconductor. All the exposure limits are taken from Occupational Safety and Health Administration (OSHA) regulations. This study helps to increase the primary care provider’s knowledge of hazardous substances such as arsenic in the environment and to promote the adoption of medical practices that aid in the evaluation and care of potentially exposed patients. Keywords Iron-based superconductor; toxicity; arsenic, exposure limits, extracted Introduction The recent discovery on the new class of iron-based superconductor has broken the tyranny of copper. The innovation of 26 K transition temperature (Tc) in fluorine-doped LaFeAsO superconductor [1] marked the beginning of worldwide efforts to investigate this new family of superconductors. After several efforts the Tc has reached 55 K by replacement of Lanthanum (La) by other rare earth elements [2-4]. -

United States Patent Office Patented Dec
3,294,827 United States Patent Office Patented Dec. 27, 1966 1. 2 3,294,827 which will remain inert during the preparative procedure. QUATERNARY AMMONIUMMETAL COMPOUNDS The term "capryly' used herein refers to the hydrocarbon Ronald R. Swanson, Minneapolis, Minn., assignor to radicals (octyl and decyl) derived from a mixture of General Mills, Inc., a corporation of Delaware caprylic acid and capric acid. No Drawing. Filed Sept. 26, 1961, Ser. No. 140,691 Another method for preparing the compounds of the 12 Claims. (CI. 260-429) present invention is by contacting the quaternary ammo nium starting material with a simple metallic salt. The This invention relates to novel quaternary ammonium quaternary and the metal salt are combined in a mutual compounds. solvent and, upon removing the solvent, the desired qua It is an object of this invention to provide novel qua 10 ternary ammonium metal compound is prepared. For ternary ammonium metal compounds. It is another ob example, if it is desired to prepare a quaternary ammo ject of this invention to provide the novel process for nium compound having an anion MnCl4, this can be the preparation of quaternary ammonium metal com done by contacting quaternary ammonium starting mate pounds. Other objects will appear hereinafter. rial with manganese chloride in a mutual solvent such as The objects of this invention are accomplished by a 5 ether or isopropanol. While the product must be com fatty quaternary ammonium metal compound wherein the pletely soluble in the chosen solvent, it is not generally anion of said quaternary compound is an inorganic anion necessary that both the starting quaternary compound and containing a metal of Group VIIB of the Periodic Table. -

Atomic Structure and Non-Electronic Properties of Semiconductors
SEMICONDUCTORS VOLUME 32, NUMBER 7 JULY 1998 ATOMIC STRUCTURE AND NON-ELECTRONIC PROPERTIES OF SEMICONDUCTORS Indium layers in low-temperature gallium arsenide: structure and how it changes under annealing in the temperature range 500–700 °C N. A. Bert, A. A. Suvorova, and V. V. Chaldyshev A. I. Ioffe Physicotechnical Institute, Russian Academy of Sciences, 194021 St. Petersburg, Russia Yu. G. Musikhin A. I. Ioffe Physicotechnical Institute, Russian Academy of Sciences, 194021 St. Petersburg, Russia; Max Planck Institut fu¨r Mikrostrukturphysik, D-5120 Halle, Germany V. V. Preobrazhenski , M. A. Putyato, and B. R. Semyagin Institute of Semiconductor Physics, Russian Academy of Sciences, Siberian Branch, 630090 Novosibirsk, Russia R. Werner Max Planck Institut fu¨r Mikrostrukturphysik, D-05120 Halle, Germany ~Submitted December 29, 1997; accepted for publication December 31, 1997! Fiz. Tekh. Poluprovodn. 32, 769–774 ~July 1998! Transmission electron microscopy is used to study the microstructure of indium d layers in GaAs~001! grown by molecular beam epitaxy at low temperature ~200 °C!. This material, referred to as LT-GaAs, contains a high concentration ('1020 cm23) of point defects. It is established that when the material is d-doped with indium to levels equivalent to 0.5 or 1 monolayer ~ML!, the roughness of the growth surface leads to the formation of InAs islands with characteristic lateral dimensions ,10 nm, which are distributed primarily within four adjacent atomic layers, i.e., the thickness of the indium-containing layer is 1.12 nm. Subsequent annealing, even at relatively low temperatures, leads to significant broadening of the indium- containing layers due to the interdiffusion of In and Ga, which is enhanced by the presence of a high concentration of point defects, particularly VGa ,inLT-GaAs. -

Relationship Between the Molecular Conformation of Choline Aryl Ethers and Nicotine-Like Stimulant Activity
Br. J. Pharmac. Chemother. (1968), 32, 113-126. RELATIONSHIP BETWEEN THE MOLECULAR CONFORMATION OF CHOLINE ARYL ETHERS AND NICOTINE-LIKE STIMULANT ACTIVITY BY E. R. CLARK, P. M. DAWES* ANi) S. G. WILLIAMS t From the Department of Pharmacology, School of Medicine, University of Leeds, Leeds 2 (Received August 7, 1967) The introduction of one or more methyl substituents into the structure of choline phenyl ether bromide (TMl) may result in large changes, both qualitative and quantitative, in pharmacological action. Thus, whereas choline o-tolyl ether bromide (o-Me-TM1), and a-methylcholine phenyl ether bromide (a-Me-TMI) are potent ganglion stimulants, being respectively one half and twice as active as choline phenyl ether (Hunt & Renshaw, 1936), choline 2,6-xylyl ether bromide (xylocholine) and /8-methylcholine phenyl ether bromide (P-Me-TMl) are devoid of ganglion stimulant activity (Hey, 1952; Clark & Jana, 1966), and xylocholine possesses adrenergic neurone blocking activity. An examination of molecular models of xylocholine and choline phenyl ether suggested that the difference in biological action might be steric in origin. Xylocholine is very sterically hindered, rotation about its phenyl-oxygen bond is virtually completely inhibited, and the plane containing the oxygen valencies is orthogonal to the plane of the benzene ring. In contrast choline phenyl ether is completely flexible and able to adopt different conformations in which the angle between the plane of the benzene ring and the plane containing the oxygen valencies (" angle-of-twist") varies between 00 and 900. p-Me-TMl lies between these two extremes; some degree of rotation about the phenyl- oxygen bond is possible but steric hindrance occurs between the o-hydrogens and the ,B-methyl group, and the "angle-of-twist" is always >00.