Bilirubin Diglucuronide Synthesis by a UDP-Glucuronic Acid
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Hyperbilirubinemia
Porphyrins Porphyrins (Porphins) are cyclic tetrapyrol compounds formed by the linkage )). of four pyrrole rings through methenyl bridges (( HC In the reduced porphyrins (Porphyrinogens) the linkage of four pyrrole rings (tetrapyrol) through methylene bridges (( CH2 )) The characteristic property of porphyrins is the formation of complexes with the metal ion bound to nitrogen atoms of the pyrrole rings. e.g. Heme (iron porphyrin). Proteins which contain heme ((hemoproteins)) are widely distributed e.g. Hemoglobin, Myoglobin, Cytochromes, Catalase & Tryptophan pyrrolase. Natural porphyrins have substituent side chains on the eight hydrogen atoms numbered on the pyrrole rings. These side chains are: CH 1-Methyl-group (M)… (( 3 )) 2-Acetate-group (A)… (( CH2COOH )) 3-Propionate-group (P)… (( CH2CH2COOH )) 4-Vinyl-group (V)… (( CH CH2 )) Porphyrins with asymmetric arrangement of the side chains are classified as type III porphyrins while those with symmetric arrangement of the side chains are classified as type I porphyrins. Only types I & III are present in nature & type III series is more important because it includes heme. 1 Heme Biosynthesis Heme biosynthesis occurs through the following steps: 1-The starting reaction is the condensation between succinyl-CoA ((derived from citric acid cycle in the mitochondria)) & glycine, this reaction is a rate limiting reaction in the hepatic heme synthesis, it occurs in the mitochondria & is catalyzed by ALA synthase (Aminolevulinate synthase) enzyme in the presence of pyridoxal phosphate as a cofactor. The product of this reaction is α-amino-β-ketoadipate which is rapidly decarboxylated to form δ-aminolevulinate (ALA). 2-In the cytoplasm condensation reaction between two molecules of ALA is catalyzed by ALA dehydratase enzyme to form two molecules of water & one 2 molecule of porphobilinogen (PBG) which is a precursor of pyrrole. -

Porphyrins & Bile Pigments
Bio. 2. ASPU. Lectu.6. Prof. Dr. F. ALQuobaili Porphyrins & Bile Pigments • Biomedical Importance These topics are closely related, because heme is synthesized from porphyrins and iron, and the products of degradation of heme are the bile pigments and iron. Knowledge of the biochemistry of the porphyrins and of heme is basic to understanding the varied functions of hemoproteins in the body. The porphyrias are a group of diseases caused by abnormalities in the pathway of biosynthesis of the various porphyrins. A much more prevalent clinical condition is jaundice, due to elevation of bilirubin in the plasma, due to overproduction of bilirubin or to failure of its excretion and is seen in numerous diseases ranging from hemolytic anemias to viral hepatitis and to cancer of the pancreas. • Metalloporphyrins & Hemoproteins Are Important in Nature Porphyrins are cyclic compounds formed by the linkage of four pyrrole rings through methyne (==HC—) bridges. A characteristic property of the porphyrins is the formation of complexes with metal ions bound to the nitrogen atom of the pyrrole rings. Examples are the iron porphyrins such as heme of hemoglobin and the magnesium‐containing porphyrin chlorophyll, the photosynthetic pigment of plants. • Natural Porphyrins Have Substituent Side Chains on the Porphin Nucleus The porphyrins found in nature are compounds in which various side chains are substituted for the eight hydrogen atoms numbered in the porphyrin nucleus. As a simple means of showing these substitutions, Fischer proposed a shorthand formula in which the methyne bridges are omitted and a porphyrin with this type of asymmetric substitution is classified as a type III porphyrin. -

Bilirubin Metabolism (Lecture 8)
Bilirubin Metabolism (Lecture 8) Excretory function of bile Bile is a medium for excretion of many substances as bile pigments, cholesterol, many drugs, toxins and various organic substances as copper and zinc. These substances are then eliminated in the feces. One of these substances excreted in bile is the greenish yellow pigment bilirubin. This is a major end product of hemoglobin degradation. It’s highly soluble in all cell membranes & is also very toxic. Therefore, its excretion in the bile is one of the very important functions of the liver. FATE OF RED BLOOD CELLS Life span of RBCs in blood stream is 60-120 days. Senescent RBCs become too fragile to exist longer in the circulatory system, their cell membranes rupture and they are phagocytosed and/or lysed. Normally, lysis occurs extravascularly in the reticuloendothelial system subsequent to RBC phagocytosis. Lysis can also occur intravascularly (in blood stream). The hemoglobin is first split into globin & heme. The AA formed from breakdown of globin are stored in the body. Metabolism of bilirubin The heme ring is opened to give: 1. Free iron that is transported in the blood by transferrin and stored in the body as a reservoir for erythropoiesis. 2. Bile pigments: The 1st pigment is biliverdin but it is rapidly reduced by biliverdin reductase to free bilirubin which is gradually released into the plasma. The free bilirubin is hydrophobic, immediately combines with plasma proteins (mainly albumin and globulin) forming a water soluble compound called hemobilirubin (unconjugated bilirubin) which is rapidly transported to hepatocytes for further metabolism. Even when bound to albumin it’s called free bilirubin. -

Biochemistry I Enzymes
BIOCHEMISTRY I 3rd. Stage Lec. ENZYMES Biomedical Importance: Enzymes, which catalyze the biochemical reactions, are essential for life. They participate in the breakdown of nutrients to supply energy and chemical building blocks; the assembly of those building blocks into proteins, DNA, membranes, cells, and tissues; and the harnessing of energy to power cell motility, neural function, and muscle contraction. The vast majority of enzymes are proteins. Notable exceptions include ribosomal RNAs and a handful of RNA molecules imbued with endonuclease or nucleotide ligase activity known collectively as ribozymes. The ability to detect and to quantify the activity of specific enzymes in blood, other tissue fluids, or cell extracts provides information that complements the physician’s ability to diagnose and predict the prognosis of many diseases. Further medical applications include changes in the quantity or in the catalytic activity of key enzymes that can result from genetic defects, nutritional deficits, tissue damage, toxins, or infection by viral or bacterial pathogens (eg, Vibrio cholerae). Medical scientists address imbalances in enzyme activity by using pharmacologic agents to inhibit specific enzymes and are investigating gene therapy as a means to remedy deficits in enzyme level or function. In addition to serving as the catalysts for all metabolic processes, their impressive catalytic activity, substrate specificity, and stereospecificity enable enzymes to fulfill key roles in additional processes related to human health and well-being. Proteases and amylases augment the capacity of detergents to remove dirt and stains, and enzymes play important roles in producing or enhancing the nutrient value of food products for both humans and animals. -

NEONATAL Hb, O2-TRANSPORT & JAUNDICE: OVERVIEW
BILIRUBIN METABOLISM & JAUNDICE UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ Temple 1 Brief description of Hemoglobin (Hb) structure • Hemoglobin (Hb): Made up of 4-Subunits (Tetramer) held together by multiple non-covalent interactions; • Each subunit consist of: • Heme (Ferro-Protoporphyrin), • Globin protein; • Heme: Protoporphyrin IX and Ferrous ion (Fe2); • Globin protein folds around Heme group forming a protective Hydrophobic pocket; • Heme is the site of Oxygen binding; 2 • There are different types of Hemoglobin, with different subunits: • Foetal Hemoglobin (Hb F): 2 2 Two types of Adult Hemoglobin (Hb A): • Hb A1 represented as: 2 2 • It is the major (98%) form of Hb in adults; • Hb A2 represented as: 2 2 • It is the minor (2%) form of Hb in adults; 3 What are the major sources of Heme in humans? • RBC is major source of Heme in humans, • Life span of RBC is about 120 days, • Other sources of Heme include: • Myoglobin (Mb): Stores Oxygen in muscle cells, • Cytochromes: present in some enzymes, • Catalase: an enzyme, 4 What normally happens to RBC after 120 days? • RBC is destroyed mainly in Reticuloendothelial system (Extra-vascular system: Spleen and Liver); • Daily turnover of Hb is about 6 g/day; • Hb is broken down, • Globin protein is hydrolyzed to amino acids, • Protoporphyrin Ring in Heme is Hydrophobic, thus must be made soluble before it is excreted, • Ferrous ion is removed and stored in Iron pool, for reuse, • Protoporphyrin ring is metabolized -
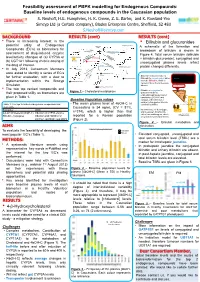
Baseline Levels of Endogenous Compounds in the Caucasian Population S
Feasibility assessment of PBPK modelling for Endogenous Compounds: Baseline levels of endogenous compounds in the Caucasian population S. Neuhoff, H.E. Humphries, H. K. Crewe, Z. E. Barter, and K. Rowland-Yeo Simcyp Ltd (a Certara company), Blades Enterprise Centre, Sheffield, S2 4SU [email protected] BACKGROUND RESULTS (cont) RESULTS (cont) • There is increasing interest in the • Bilirubin and glucuronides 27-Hydroxycholesterol potential utility of Endogenous Cholesterol 1g/day A schematic of the formation and Compounds (EC’s) as biomarkers for CYP7A1 (liver) 500 mg/day CYP27A1 (many tissues) breakdown of bilirubin is shown in assessment of drug-induced enzyme 19 mg/day Figure 4. Total serum bilirubin (bilirubin 7α-Hydroxycholesterol level/activity changes of (a) CYP3A or CYP3A4 + bilirubin glucuronide), conjugated and Bile Acids (b) UGT1A1 following chronic dosing of 4-Hydroxycholesterol unconjugated plasma levels reflect the drug of interest. CYP27A1 (liver and extrahepatic) 24S-Hydroxycholesterol protein changes differently. • In July 2013, Consortium Members Pregnenolone were asked to identify a series of EC’s 4,27-Dihydroxycholesterol Macrophage CYP27A1 • Biliverdin reductase reduces Heme Hemeoxygenase for further evaluation, with a view to 4,24-Dihydroxycholesterol Biliverdin to unconjugated, water- Biliverdin 4,7α-Dihydroxycholesterol insoluble Bilirubin, which is Biliverdin reductase implementation within the Simcyp Bilirubin carried in blood bound to serum Unconjugated bilirubin Simulator. albumin complexed with albumin 3 -

Bilirubin PDF 12Days
Bilirubin Physiology – WYNTKFTB • Bilirubin metabolism – Unconjugated – Conjugation – Excretion • Enterohepatic circulation • Implication of bilirubin metabolism – Urine – Stool • Understand the metabolic pathway as related to the disease state of JAUNDICE Don’t make me come up there and steal your google machines! BR - Alb Thank you. BG the Bilirubin an overview by howard sachs Slo-Motion Instant Replay… Heme oxygenase Biliverdin Reductase Heme oxygenase Biliverdin Reductase Bilirubin Albumin (unconjugated) …not water soluble… Unconjugated Bilirubin (‘Indirect’) Key points: • ‘Attached to albumin’: too big to pass through glomerular filtration barrier. • Intravascular: Hemoglobin ® urine • Unconjugated: not water soluble. How can we get too much unconjugated bilirubin in the serum? 1. Hemolysis • Elevated indirect bilirubin is used as a diagnostic marker (with LDH, ¯ haptoglobin) 2. Conjugation defect: inherited (Gilbert’s, Crigler-Najjar) Multidrug resistance associated protein Bilirubin binds to cytosolic binding protein, glutathione s-transferase (GST) for transport to ER Conjugation of bilirubin is catalyzed by bilirubin-uridinediphosphate (UDP)- glucuronosyltransferase (UGT1A1). Product is bilirubin diglucuronide. UDPGA is the donor sugar Multidrug resistance Endoplasmic reticulum associated protein Bilirubin diglucuronide Conjugated (direct) Bilirubin binds to cytosolic binding protein, glutathione s-transferase (GST) for transport to ER Conjugation of bilirubin is catalyzed by bilirubin-uridinediphosphate (UDP)- glucuronosyltransferase -

Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors
Pharmaceutics 2011 , 3, 615-635; doi:10.3390/pharmaceutics3030615 OPEN ACCESS pharmaceutics ISSN 1999-4923 www.mdpi.com/journal/pharmaceutics Review Transporter-Mediated Drug Interaction Strategy for 5-Aminolevulinic Acid (ALA)-Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors Toshihisa Ishikawa 1,*, Kenkichi Takahashi 2, Naokado Ikeda 2, Yoshinaga Kajimoto 2, Yuichiro Hagiya 3, Shun-ichiro Ogura 3, Shin-ichi Miyatake 2 and Toshihiko Kuroiwa 2 1 Omics Science Center, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, 230-0045, Japan 2 Department of Neurosurgery, Osaka Medical College, Osaka 569-8686, Japan 3 Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama 226-8501, Japan * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel.: +81-45-503-9222; Fax: +81-45-503-9216. Received: 19 July 2011; in revised form: 16 August 2011 / Accepted: 9 September 2011 / Published: 14 September 2011 Abstract: Photodynamic diagnosis (PDD) is a practical tool currently used in surgical operation of aggressive brain tumors, such as glioblastoma. PDD is achieved by a photon-induced physicochemical reaction which is induced by excitation of protoporphyrin IX (PpIX) exposed to light. Fluorescence-guided gross-total resection has recently been developed in PDD, where 5-aminolevulinic acid (ALA) or its ester is administered as the precursor of PpIX. ALA induces the accumulation of PpIX, a natural photo-sensitizer, in cancer cells. Recent studies provide evidence that adenosine triphosphate (ATP)-binding cassette (ABC) transporter ABCG2 plays a pivotal role in regulating the cellular accumulation of porphyrins in cancer cells and thereby affects the efficacy of PDD. -

Bilirubin Secretion, Jaundice and Evaluation of Liver Function
Bilirubin Secretion, Jaundice and Evaluation of Liver Function Howard J. Worman, M. D. Evaluation of Liver Disease and Hepatic Function History Physical Examination Laboratory Tests Sometimes Radiological/Nuclear Medicine Sometimes Liver Biopsy 1 Jaundice occurs as a result of excess bilirubin in the blood. It is a hallmark of liver disease but not always present in liver disease. Jaundice occurs when the liver fails to adequately secrete bilirubin from the blood into the bile. To understand how jaundice occurs, you must first understand bilirubin synthesis, metabolism and secretion. 2 Heme Oxygenase Schuller et al. Nature Structural Biology 6, 860 - 867 (1999) Bilivirdin Reductase Kikuchi et al. Nature Structural Biology 8, 221 - 225 (2001) 3 Bilirubin is frequently depicted as a linear tetrapyrrole. However, intramolecular hydrogen bonding fixes it in a rigid structure that blocks exposure of its polar groups to aqueous solvents, making it very insoluble in blood. 4 Bilirubin in Blood is Bound to Albumin: Uptake into Hepatocyte at Basolateral (Sinusoidal) Membrane Some OATP-C? bilirubin stored in cytosol bound to proteins Bilurubin UDP-glucuronosyltransferase is localized to the endo- plasmic reticulum; it catalyzes conjugation to a diglucuronide, making it more water soluble. A: Labeling of periphery of cell hepatocyte nucleus B: Labeling of ER with antibody to UDP-glucuronosyltransferase 5 Alternative RNA splicing of different first exons of UGT1 gives different isoforms with different substrate specificities, some for bilirubin and others -

(12) Patent Application Publication (10) Pub. No.: US 2001/0034023 A1 Stanton, JR
US 2001.0034O23A1. (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0034023 A1 Stanton, JR. et al. (43) Pub. Date: Oct. 25, 2001 (54) GENE SEQUENCE VARIATIONS WITH Related U.S. Application Data UTILITY IN DETERMINING THE (63) Continuation-in-part of application No. 09/710,467, TREATMENT OF DISEASE, INGENES filed on Nov. 8, 2000, which is a continuation-in-part RELATING TO DRUG PROCESSING of application No. 09/696,482, filed on Oct. 24, 2000. Non-provisional of provisional application No. 60/131,334, filed on Apr. 26, 1999. Non-provisional (76) Inventors: Vincent P. Stanton JR., Belmont, MA of provisional application No. 60/139,440, filed on (US); Martin Zillmann, Shrewsbury, Jun. 15, 1999. MA (US) (30) Foreign Application Priority Data Correspondence Address: Jan. 20, 2000 (WO)........................... PCT/USOO/O1392 EDWARD O. KRUESSER BROBECK PHLEGER & HARRISON Publication Classification 12390 EL CAMINO REAL (51) Int. Cl. ............................. C12O 1/68; G06F 19/00 SAN DIEGO, CA 92130 (US) (52) U.S. Cl. ................................................... 435/6; 702/20 (57) ABSTRACT (21) Appl. No.: 09/733,000 Methods for identifying and utilizing variances in genes relating to efficacy and Safety of medical therapy and other aspects of medical therapy are described, including methods (22) Filed: Dec. 7, 2000 for Selecting an effective treatment. US 2001/0034023 A1 Oct. 25, 2001 GENE SEQUENCE WARIATIONS WITH UTILITY 0005 For some drugs, over 90% of the measurable IN DETERMINING THE TREATMENT OF interSubject variation in Selected pharmacokinetic param DISEASE, INGENES RELATING TO DRUG eters has been shown to be heritable. For a limited number PROCESSING of drugs, DNA sequence variances have been identified in Specific genes that are involved in drug action or metabo RELATED APPLICATIONS lism, and these variances have been shown to account for the 0001. -

Inherited Disorders of Bilirubin Clearance N
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Hofstra Northwell Academic Works (Hofstra Northwell School of Medicine) Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2015 Inherited disorders of bilirubin clearance N. Memon B. I. Weinberger Zucker School of Medicine at Hofstra/Northwell T. Hegyi L. M. Aleksunes Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Pediatrics Commons Recommended Citation Memon N, Weinberger B, Hegyi T, Aleksunes L. Inherited disorders of bilirubin clearance. 2015 Jan 01; 79(3):Article 2776 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/2776. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. For more information, please contact [email protected]. HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Pediatr Manuscript Author Res. Author manuscript; Manuscript Author available in PMC 2016 April 05. Published in final edited form as: Pediatr Res. 2016 March ; 79(3): 378–386. doi:10.1038/pr.2015.247. Inherited Disorders of Bilirubin Clearance Naureen Memon1,*, Barry I Weinberger2, Thomas Hegyi1, and Lauren M Aleksunes3 1Department of Pediatrics, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA 2Department of Pediatrics, Cohen Children’s Medical Center of New York, New Hyde Park, NY, USA 3Department of Pharmacology and Toxicology, Rutgers University, Piscataway, NJ, USA Abstract Inherited disorders of hyperbilirubinemia may be caused by increased bilirubin production or decreased bilirubin clearance. -

Novel Pharmacogenomic Markers of Irinotecan-Induced Severe Toxicity in Metastatic Colorectal Cancer Patients
Novel pharmacogenomic markers of irinotecan-induced severe toxicity in metastatic colorectal cancer patients Thèse Siwen Sylvialin Chen Doctorat en sciences pharmaceutiques Philosophiae doctor (Ph.D.) Québec, Canada © Siwen Sylvialin Chen, 2015 ii Résumé L’irinotécan est un agent de chimiothérapie largement utilisé pour le traitement de tumeurs solides, particulièrement pour le cancer colorectal métastatique (mCRC). Fréquemment, le traitement par l’irinotécan conduit à la neutropénie et la diarrhée, des effets secondaires sévères qui peuvent limiter la poursuite du traitement et la qualité de vie des patients. Plusieurs études pharmacogénomiques ont évalué les risques associés à la chimiothérapie à base d’irinotécan, en particulier en lien avec le gène UGT1A, alors que peu d’études ont examiné l’impact des gènes codant pour des transporteurs. Par exemple, le marqueur UGT1A1*28 a été associé à une augmentation de 2 fois du risque de neutropénie, mais ce marqueur ne permet pas de prédire la toxicité gastrointestinale ou l’issue clinique. L’objectif de cette étude était de découvrir de nouveaux marqueurs génétiques associés au risque de toxicité induite par l’irinotécan, en utilisant une stratégie d’haplotype/SNP-étiquette permettant de maximiser la couverture des loci génétiques ciblés. Nous avons analysé les associations génétiques des loci UGT1 et sept gènes codants pour des transporteurs ABC impliqués dans la pharmacocinétique de l’irinotécan, soient ABCB1, ABCC1, ABCC2, ABCC5, ABCG1, ABCG2 ainsi que SLCO1B1. Les profils de 167 patients canadiens atteints de mCRC sous traitement FOLFIRI (à base d’irinotécan) ont été examinés et les marqueurs significatifs ont par la suite été validés dans une cohorte indépendante de 250 patients italiens.