Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glycosides in Lemon Fruit
Food Sci. Technol. Int. Tokyo, 4 (1), 48-53, 1998 Characteristics of Antioxidative Flavonoid Glycosides in Lemon Fruit Yoshiaki MIYAKE,1 Kanefumi YAMAMOT0,1 Yasujiro MORIMITSU2 and Toshihiko OSAWA2 * Central Research Laboratory of Pokka Corporation, Ltd., 45-2 Kumanosyo, Shikatsu-cho, Nishikasugai-gun, Aichi 481, Japan 2Department of Applied Biological Sciences, Nagoya University, Nagoya 46401, Japan Received June 12, 1997; Accepted September 27, 1997 We investigated the antioxidative flavonoid glycosides in the peel extract of lemon fruit (Citrus limon). Six flavanon glycosides: eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, and neohesperidin, and three flavone glycosides: diosmin, 6~-di- C-p-glucosyldiosmin (DGD), and 6- C-p-glucosyldiosmin (GD) were identified by high- performance liquid chromatography (HPLC) analysis. Their antioxidative activity was examined using a linoleic acid autoxidation system. The antioxidative activity of eriocitrin, neoeriocitrin and DGD was stronger than that of the others. Flavonoid glycosides were present primarily in the peel of lemon fruit. There was only a small difference in the content of the flavonoid glycosides of the lemon fruit juice from various sources and varieties. Lemon fruit contained abundant amounts of eriocitrin and hesperidin and also contained narirutin, diosmin, and DGD, but GD, neoeriocitrin, naringin, and neohesperidin were present only in trace amounts. The content of DGD, GD, and eriocitrin was especially abundant in lemons and limes; however, they were scarcely found in other citrus fruits. The content of flavonoid compounds in lemon juice obtained by an in-line extractor at a juice factory was more abundant than that obtained by hand-squeezing. These compounds were found to be stable even under heat treatment conditions (121'C, 15 min) in acidic solution. -

Absorption and Pharmacokinetics of Grapefruit Flavanones in Beagles
Downloaded from British Journal of Nutrition (2007), 98, 86–92 doi: 10.1017/S0007114507707262 q The Authors 2007 https://www.cambridge.org/core Absorption and pharmacokinetics of grapefruit flavanones in beagles Maria de Lourdes Mata-Bilbao1, Cristina Andre´s-Lacueva1, Elena Roura1, Olga Ja´uregui2, Elvira Escribano3, 4 1 Celina Torre and Rosa Maria Lamuela-Ravento´s * . IP address: 1Department of Nutrition and Food Science, CerTA, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, Barcelona, Spain 2 Scientific and Technical Services, University of Barcelona, Barcelona, Spain 170.106.35.229 3Biopharmaceutics and Pharmacokinetics Unit, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, Barcelona, Spain 4Affinity Pet-care, Barcelona, Spain , on (Received 25 August 2006 – Revised 8 January 2007 – Accepted 24 January 2007) 29 Sep 2021 at 02:36:36 The present study evaluated the pharmacokinetics of three different grapefruit flavanone forms in dog plasma and demonstrated their absorption after an oral intake of a grapefruit extract; pharmacokinetic parameters of these forms were also determined. Ten healthy beagles were adminis- tered 70 mg citrus flavonoids as a grapefruit extract contained in capsules, while two additional dogs were used as controls and given an excipient. The grapefruit flavanone naringin, along with its metabolites naringenin and naringenin glucuronide, was detected in dog plasma. Blood , subject to the Cambridge Core terms of use, available at samples were collected between 0 and 24 h after administration of the extract. Naringin reached its maximun plasma concentration at around 80 min, whereas naringenin and naringenin glucuronide reached their maximun plasma concentrations at around 20 and 30 min, respectively. -

A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice
molecules Communication A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice Santina Chiechio 1,2,* , Magda Zammataro 1, Massimo Barresi 1, Margherita Amenta 3 , Gabriele Ballistreri 3 , Simona Fabroni 3 and Paolo Rapisarda 3 1 Section of Pharmacology, Department of Drug and Health Sciences, University of Catania, 95123 Catania, Italy; [email protected] (M.Z.); [email protected] (M.B.) 2 Oasi Research Institute IRCCS, 94018 Troina, Italy 3 Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria, Centro di Ricerca Olivicoltura, Frutticoltura e Agrumicoltura, 95024 Acireale, Italy; [email protected] (M.A.); [email protected] (G.B.); [email protected] (S.F.); [email protected] (P.R.) * Correspondence: [email protected] Abstract: Citrus fruits are a rich source of high-value bioactive compounds and their consumption has been associated with beneficial effects on human health. Red (blood) oranges (Citrus sinensis L. Osbeck) are particularly rich in anthocyanins (95% of which are represented by cyanidin-3- glucoside and cyanidin-3-6”-malonyl-glucoside), flavanones (hesperidin, narirutin, and didymin), Citation: Chiechio, S.; Zammataro, and hydroxycinnamic acids (caffeic acid, coumaric acid, sinapic, and ferulic acid). Lemon fruit (Citrus M.; Barresi, M.; Amenta, M.; limon) is also rich in flavanones (eriocitrin, hesperidin, and diosmin) and other polyphenols. All Ballistreri, G.; Fabroni, S.; Rapisarda, of these compounds are believed to play a very important role as dietary antioxidants due to their P. A Standardized Extract Prepared ability to scavenge free radicals. -

Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges
molecules Article Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck) Shengyu Liu 1, Linping Hu 1, Dong Jiang 2 and Wanpeng Xi 1,3,* 1 College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400716, China; [email protected] (S.L.); [email protected] (L.H.) 2 Citrus Research Institute, Chinese Academy of Agricultural Sciences, Chongqing 400712, China; [email protected] 3 Key Laboratory of Horticulture Science for Southern Mountainous Regions, Ministry of Education, Chongqing 400715, China * Correspondence: [email protected]; Tel.: +86-23-68250483; Fax: +86-23-68251274 Received: 20 March 2019; Accepted: 4 May 2019; Published: 6 May 2019 Abstract: To investigate the effect of post-harvest light irradiation on the accumulation of flavonoids and limonoids, harvested Newhall navel oranges were continuously exposed to light-emitting diode (LED) and ultraviolet (UV) light irradiation for 6 days, and the composition and content of flavonoids and limonoids in the segments were determined using UPLC-qTOF-MS at 0, 6, and 15 days after harvest. In total, six polymethoxylated flavonoids (PMFs), five flavone-O/C-glycosides, seven flavanone-O-glycosides, and three limonoids were identified in the segments. The accumulation of these components was altered by light irradiation. Red and blue light resulted in higher levels of PMFs during exposure periods. The accumulation of PMFs was also significantly induced after white light, UVB and UVC irradiation were removed. Red and UVC irradiation induced the accumulation of flavone and flavanone glycosides throughout the entire experimental period. -
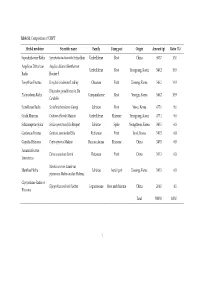
Ratio (%) Saposhnikoviae Ra
Table S1. Composition of CSBPT Herbal medicine Scientific name Family Using part Origin Amount (g) Ratio (%) Saposhnikoviae Radix Saposhnikovia divaricate Schischkin Umbelliferae Root China 500.7 10.0 Angelicae Dahuricae Angelica dahurica Bentham et Umbelliferae Root Yeongyang, Korea 546.2 10.9 Radix Hooker F. Forsythiae Fructus Forsythia viridissima Lindley Oleaceae Fruit Uiseong, Korea 546.2 10.9 Platycodon grandiflorum A. De Platycodonis Radix Campanulaceae Root Yeongju, Korea 546.2 10.9 Candolle Scutellariae Radix Scutellaria baicalensis Georgi Labiatae Root Yeosu, Korea 477.1 9.6 Cnidii Rhizoma Cnidium officinale Makino Umbelliferae Rhizome Yeongyang, Korea 477.1 9.6 Schizonepetae Spica Schizonepeta tenuifolia Briquet Labiatae Spike Yeongcheon, Korea 340.5 6.8 Gardeniae Fructus Gardenia jasminoides Ellis Rubiaceae Fruit Imsil, Korea 340.5 6.8 Coptidis Rhizoma Coptis japonica Makino Ranunculaceae Rhizome China 340.5 6.8 Aurantii Fructus Citrus aurantium Linné Rutaceae Fruit China 340.5 6.8 Immaturus Mentha arvensis Linné var. Menthae Herba Labiatae Aerial part Uiseong, Korea 340.5 6.8 piperascens Malinvaud ex Holmes Glycyrrhizae Radix et Glycyrrhiza uralensis Fischer Leguminosae Root and rhizome China 204.0 4.1 Rhizoma Total 5000.0 100.0 1 Table 2. Chromatographic conditions for simultaneous quantification of compounds 1–18 in CSBPT. Chromatographic parameter Column SunFire C18 analytical column (250 × 4.6 mm, 5 μm) Detector PDA (235, 250, 280, 310, and 345 nm) Flow rate (mL/min) 1.0 Injection volume (μL) 10.0 Column temperature (°C) 40.0 A: 0.1% (v/v) aqueous formic acid Mobile phase B: 0.1% (v/v) formic acid in acetonitrile Time (min) A (%) B (%) 0 95 5 40 40 60 Gradient elution 50 5 95 55 5 95 60 95 5 70 95 5 2 Table S3. -

Flavonoid-Rich Orange Juice Is Associated with Acute
Eur J Nutr (2016) 55:2021–2029 DOI 10.1007/s00394-015-1016-9 ORIGINAL CONTRIBUTION Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males Mudi H. Alharbi2 · Daniel J. Lamport1 · Georgina F. Dodd1 · Caroline Saunders3 · Laura Harkness3 · Laurie T. Butler1 · Jeremy P. E. Spencer2 Received: 26 February 2015 / Accepted: 5 August 2015 / Published online: 18 August 2015 © The Author(s) 2015. This article is published with open access at Springerlink.com Abstract speed was significantly better following the FR drink com- Purpose Epidemiological evidence suggests that chronic pared to the placebo. The effects of objective cognitive consumption of fruit-based flavonoids is associated with function were supported by significant benefits for subjec- cognitive benefits; however, the acute effects of flavonoid- tive alertness following the FR drink relative to the placebo. rich (FR) drinks on cognitive function in the immediate Conclusions These data demonstrate that consumption of postprandial period require examination. The objective was FR orange juice can acutely enhance objective and subjec- to investigate whether consumption of FR orange juice is tive cognition over the course of 6 h in healthy middle-aged associated with acute cognitive benefits over 6 h in healthy adults. middle-aged adults. Methods Males aged 30–65 consumed a 240-ml FR Keywords Flavonoids · Flavanones · Cognition · orange juice (272 mg) and a calorie-matched placebo in a Cognitive function · Orange juice randomized, double-blind, counterbalanced order on 2 days separated by a 2-week washout. Cognitive function and subjective mood were assessed at baseline (prior to drink Introduction consumption) and 2 and 6 h post consumption. -

Review Article Chemistry and Biological Activities of Flavonoids: an Overview
Hindawi Publishing Corporation The Scientific World Journal Volume 2013, Article ID 162750, 16 pages http://dx.doi.org/10.1155/2013/162750 Review Article Chemistry and Biological Activities of Flavonoids: An Overview Shashank Kumar and Abhay K. Pandey Department of Biochemistry, University of Allahabad, Allahabad 211002, India Correspondence should be addressed to Abhay K. Pandey; [email protected] Received 24 August 2013; Accepted 7 October 2013 Academic Editors: K. P. Lu and J. Sastre Copyright © 2013 S. Kumar and A. K. Pandey. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. There has been increasing interest in the research on flavonoids from plant sources because of their versatile health benefits reported in various epidemiological studies. Since flavonoids are directly associated with human dietary ingredients and health, there is need to evaluate structure and function relationship. The bioavailability, metabolism, and biological activity of flavonoids depend upon the configuration, total number of hydroxyl groups, and substitution of functional groups about their nuclear structure. Fruits and vegetables are the main dietary sources of flavonoids for humans, along with tea and wine. Most recent researches have focused on the health aspects of flavonoids for humans. Many flavonoids are shown to have antioxidative activity, free radical scavenging capacity, coronary heart disease prevention, hepatoprotective, anti-inflammatory, and anticancer activities, while some flavonoids exhibit potential antiviral activities. In plant systems, flavonoids help in combating oxidative stress and act as growth regulators. For pharmaceutical purposes cost-effective bulk production of different types of flavonoids has been made possible with the help of microbial biotechnology. -

Preclinical Evidence for the Pharmacological Actions of Naringin: a Review
Reviews 437 Preclinical Evidence for the Pharmacological Actions of Naringin: A Review Authors Saurabh Bharti, Neha Rani, Bhaskar Krishnamurthy, Dharamvir Singh Arya Affiliation Department of Pharmacology, All India Institute of Medical Sciences, New Delhi, India Key words Abstract BDNF: brain-derived neurotrophic factor l" naringin ! BMP: bone morphogenetic protein l" flavonoid Naringin, chemically 4′,5,7- trihydroxyflavanone- DMBA: 7,12-dimethylbenz[a]anthracene l" antioxidant 7-rhamnoglucoside, is a major flavanone glyco- DNFB: 2,4-dinitrofluorobenzene l" anti‑inflammatory side obtained from tomatoes, grapefruits, and DPP: dipeptidyl peptidase l" atherosclerosis l" diabetes mellitus many other citrus fruits. It has been experimen- DSS: dextran sodium sulphate l" neurological disorders tally documented to possess numerous biological EGF: epidermal growth factor l" cardiovascular disorders properties such as antioxidant, anti-inflamma- eNOS: endothelial nitric oxide synthase l" hepatoprotection tory, and antiapoptotic activities. In vitro and in ER: estrogen receptor l" nephroprotection vivo studies have further established the useful- ERK: extracellular signal-regulated kinase l" bone diseases ness of naringin in various preclinical models of FRAP: ferric reducing antioxidant power l" gastrointestinal diseases atherosclerosis, cardiovascular disorders, diabetes GSK: glycogen synthase kinase l" metabolic syndrome l" dentistry mellitus, neurodegenerative disorders, osteopo- hDuox2: human dual oxidase 2 l" cancer rosis, and rheumatological -

Cloning, Heterologous Expression in Yeast, and Biochemical
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2012 Cloning, Heterologous Expression in Yeast, and Biochemical Characterization of Recombinant Putative Glucosyltransferase Clones 9 and 11 from Grapefruit (Citrus paradisi) Anye Wamucho East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Biology Commons Recommended Citation Wamucho, Anye, "Cloning, Heterologous Expression in Yeast, and Biochemical Characterization of Recombinant Putative Glucosyltransferase Clones 9 and 11 from Grapefruit (Citrus paradisi)" (2012). Electronic Theses and Dissertations. Paper 1214. https://dc.etsu.edu/etd/1214 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Cloning, Heterologous Expression in Yeast, and Biochemical Characterization of Recombinant Putative Glucosyltransferase Clones 9 and 11 from Grapefruit (Citrus paradisi) _____________________ A thesis presented to The faculty of the Department of Biological Sciences East Tennessee State University in partial fulfillment of the requirement for the degree Master of Science in Biology _____________________ by Anye Wamucho May 2012 _____________________ Cecilia A. McIntosh, PhD, Chair Dhirendra Kumar, PhD Daniel K. Owens, PhD Keywords: Flavonoids, Phenolics, Citrus paradisi, Glucosyltransferase, PSPG Box ABSTRACT Cloning, Heterologous Expression in Yeast, and Biochemical Characterization of Recombinant Putative Glucosyltransferase Clones 9 and 11 from Grapefruit (Citrus paradisi) by Anye Wamucho Flavonoids are plant secondary metabolites that play diverse roles in plants and human health. -

Antioxidants
antioxidants Article Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies Giacomo Pepe 1, Emanuela Salviati 1,2 , Shara Francesca Rapa 1, Carmine Ostacolo 3 , Stella Cascioferro 4 , Michele Manfra 5, Giuseppina Autore 1 , Stefania Marzocco 1,* and Pietro Campiglia 1,6,* 1 Department of Pharmacy, University of Salerno, 84084 Fisciano, Italy; [email protected] (G.P.); [email protected] (E.S.); [email protected] (S.F.R.); [email protected] (G.A.) 2 PhD Program in Drug Discovery and Development, University of Salerno, 84084 Fisciano, Italy 3 Department of Pharmacy, University of Naples Federico II, 80131 Naples, Italy; [email protected] 4 Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche (STEBICEF), University of Palermo, 90123 Palermo, Italy; [email protected] 5 Department of Science, University of Basilicata, Viale dell’Ateneo Lucano 10, 85100 Potenza, Italy; [email protected] 6 European Biomedical Research Institute of Salerno, 84125 Salerno, Italy * Correspondence: [email protected] (S.M.); [email protected] (P.C.); Tel.: +39-089-96-9250 (S.M.); +39-089-96-9242 (P.C.) Received: 17 April 2020; Accepted: 1 May 2020; Published: 2 May 2020 Abstract: The interest towards nutraceuticals able to counteract drug side effects is continuously growing in current chemotherapeutic protocols. In the present study, we demonstrated that smoothies containing mixtures of Citrus sinensis and Vitis vinifera L. cv. Aglianico N, two typical fruits of the Mediterranean diet, possess bioactive polyphenols that protect cardiomyocytes against doxorubicin-induced oxidative stress. The polyphenolic extracts isolated from Citrus sinensis- and Vitis vinifera-based functional smoothies were deeply characterized by Liquid Chromatography-Mass Spectrometry methods. -

USDA Database for the Flavonoid Content of Selected Foods
USDA Database for the Flavonoid Content of Selected Foods Release 3.1 Prepared by Seema Bhagwat, David B. Haytowitz, and Joanne M. Holden (ret.) Nutrient Data Laboratory Beltsville Human Nutrition Research Center Agricultural Research Service U.S. Department of Agriculture December 2013 U.S. Department of Agriculture Agricultural Research Service Beltsville Human Nutrition Research Center Nutrient Data Laboratory 10300 Baltimore Avenue Building 005, Room 107, BARC-West Beltsville, Maryland 20705 Tel. 301-504-0630, FAX: 301-504-0632 E-Mail: [email protected] Web site: http://www.ars.usda.gov/nutrientdata Table of Contents Release History ............................................................................................. i Suggested Citation: ...................................................................................... ii Documentation .............................................................................................1 Subclasses of flavonoids and selected compounds .................................2 Methods and Procedures used to generate the database ........................2 Data Evaluation ........................................................................................4 Flavonoid Individual Data Table ...............................................................5 Sources of Data ...........................................................................................6 Format of the Tables ....................................................................................7 Food Description -

The Therapeutic Potential of Naringenin: a Review of Clinical Trials
pharmaceuticals Review The Therapeutic Potential of Naringenin: A Review of Clinical Trials Bahare Salehi 1 , Patrick Valere Tsouh Fokou 2 , Mehdi Sharifi-Rad 3,*, Paolo Zucca 4,* , Raffaele Pezzani 5,6,* , Natália Martins 7,8,* and Javad Sharifi-Rad 9,10,* 1 Student Research Committee, School of Medicine, Bam University of Medical Sciences, Bam 44340847, Iran; [email protected] 2 Antimicrobial and Biocontrol Agents Unit, Department of Biochemistry, Faculty of Science, University of Yaounde 1, Ngoa Ekelle, Annex Fac. Sci., Yaounde 812, Cameroon; [email protected] 3 Department of Medical Parasitology, Zabol University of Medical Sciences, Zabol 61663-335, Iran 4 Department of Biomedical Sciences, University of Cagliari, 09042 Cagliari, Italy 5 OU Endocrinology, Dept. Medicine (DIMED), University of Padova, via Ospedale 105, 35128 Padova, Italy 6 AIROB, Associazione Italiana per la Ricerca Oncologica di Base, 35128 Padova, Italy 7 Faculty of Medicine, University of Porto, Alameda Professor Hernâni Monteiro, 4200-319 Porto, Portugal 8 Institute for Research and Innovation in Health (i3S), University of Porto, 4200-135 Porto, Portugal 9 Zabol Medicinal Plants Research Center, Zabol University of Medical Sciences, Zabol 61615585, Iran 10 Department of Chemistry, Richardson College for the Environmental Science Complex, The University of Winnipeg, 599 Portage Avenue, Winnipeg, MB R3B 2G3, Canada * Correspondence: m.sharifi[email protected] (M.S.-R.); [email protected] (P.Z.); [email protected] (R.P.); [email protected] (N.M.); javad.sharifi[email protected] (J.S.-R.); Tel.: +98-54-322-51-790 (M.S.-R.); +39-70-675-4526 (P.Z.); + 39-049-821-3018 (R.P.); +351-22-5512100 (N.M.); +98-21-88200104 (J.S.-R.) Received: 16 November 2018; Accepted: 4 January 2019; Published: 10 January 2019 Abstract: Naringenin is a flavonoid belonging to flavanones subclass.