A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thesis of Potentially Sweet Dihydrochalcone Glycosides
University of Bath PHD The synthesis of potentially sweet dihydrochalcone glycosides. Noble, Christopher Michael Award date: 1974 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 05. Oct. 2021 THE SYNTHESIS OF POTBTTIALLY SWEET DIHYDROCHALCOITB GLYCOSIDES submitted by CHRISTOPHER MICHAEL NOBLE for the degree of Doctor of Philosophy of the University of Bath. 1974 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author.This copy of the the sis has been supplied on condition that anyone who con sults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be pub lished without the prior written consent of the author. -

Glycosides in Lemon Fruit
Food Sci. Technol. Int. Tokyo, 4 (1), 48-53, 1998 Characteristics of Antioxidative Flavonoid Glycosides in Lemon Fruit Yoshiaki MIYAKE,1 Kanefumi YAMAMOT0,1 Yasujiro MORIMITSU2 and Toshihiko OSAWA2 * Central Research Laboratory of Pokka Corporation, Ltd., 45-2 Kumanosyo, Shikatsu-cho, Nishikasugai-gun, Aichi 481, Japan 2Department of Applied Biological Sciences, Nagoya University, Nagoya 46401, Japan Received June 12, 1997; Accepted September 27, 1997 We investigated the antioxidative flavonoid glycosides in the peel extract of lemon fruit (Citrus limon). Six flavanon glycosides: eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, and neohesperidin, and three flavone glycosides: diosmin, 6~-di- C-p-glucosyldiosmin (DGD), and 6- C-p-glucosyldiosmin (GD) were identified by high- performance liquid chromatography (HPLC) analysis. Their antioxidative activity was examined using a linoleic acid autoxidation system. The antioxidative activity of eriocitrin, neoeriocitrin and DGD was stronger than that of the others. Flavonoid glycosides were present primarily in the peel of lemon fruit. There was only a small difference in the content of the flavonoid glycosides of the lemon fruit juice from various sources and varieties. Lemon fruit contained abundant amounts of eriocitrin and hesperidin and also contained narirutin, diosmin, and DGD, but GD, neoeriocitrin, naringin, and neohesperidin were present only in trace amounts. The content of DGD, GD, and eriocitrin was especially abundant in lemons and limes; however, they were scarcely found in other citrus fruits. The content of flavonoid compounds in lemon juice obtained by an in-line extractor at a juice factory was more abundant than that obtained by hand-squeezing. These compounds were found to be stable even under heat treatment conditions (121'C, 15 min) in acidic solution. -

GRAS Notice (GRN) No. 719, Orange Pomace
GRAS Notice (GRN) No. 719 https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm SAFETY EVALUATION DOSSIER SUPPORTING A GENERALLY RECOGNIZED AS SAFE (GRAS) CONCLUSION FOR ORANGE POMACE SUBMITTED BY: PepsiCo, Inc. 700 Anderson Hill Road Purchase, NY 10577 SUBMITTED TO: U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Additive Safety HFS-200 5100 Paint Branch Parkway College Park, MD 20740-3835 CONTACT FOR TECHNICAL OR OTHER INFORMATION: Andrey Nikiforov, Ph.D. Toxicology Regulatory Services, Inc. 154 Hansen Road, Suite 201 Charlottesville, VA 22911 July 3, 2017 Table of Contents Part 1. SIGNED STATEMENTS AND CERTIFICATION ...........................................................1 A. Name and Address of Notifier .............................................................................................1 B. Name of GRAS Substance ...................................................................................................1 C. Intended Use and Consumer Exposure ................................................................................1 D. Basis for GRAS Conclusion ................................................................................................2 E. Availability of Information ..................................................................................................3 Part 2. IDENTITY, METHOD OF MANUFACTURE, SPECIFICATIONS, AND PHYSICAL OR TECHNICAL EFFECT.................................................................................................4 -

Absorption and Pharmacokinetics of Grapefruit Flavanones in Beagles
Downloaded from British Journal of Nutrition (2007), 98, 86–92 doi: 10.1017/S0007114507707262 q The Authors 2007 https://www.cambridge.org/core Absorption and pharmacokinetics of grapefruit flavanones in beagles Maria de Lourdes Mata-Bilbao1, Cristina Andre´s-Lacueva1, Elena Roura1, Olga Ja´uregui2, Elvira Escribano3, 4 1 Celina Torre and Rosa Maria Lamuela-Ravento´s * . IP address: 1Department of Nutrition and Food Science, CerTA, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, Barcelona, Spain 2 Scientific and Technical Services, University of Barcelona, Barcelona, Spain 170.106.35.229 3Biopharmaceutics and Pharmacokinetics Unit, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, Barcelona, Spain 4Affinity Pet-care, Barcelona, Spain , on (Received 25 August 2006 – Revised 8 January 2007 – Accepted 24 January 2007) 29 Sep 2021 at 02:36:36 The present study evaluated the pharmacokinetics of three different grapefruit flavanone forms in dog plasma and demonstrated their absorption after an oral intake of a grapefruit extract; pharmacokinetic parameters of these forms were also determined. Ten healthy beagles were adminis- tered 70 mg citrus flavonoids as a grapefruit extract contained in capsules, while two additional dogs were used as controls and given an excipient. The grapefruit flavanone naringin, along with its metabolites naringenin and naringenin glucuronide, was detected in dog plasma. Blood , subject to the Cambridge Core terms of use, available at samples were collected between 0 and 24 h after administration of the extract. Naringin reached its maximun plasma concentration at around 80 min, whereas naringenin and naringenin glucuronide reached their maximun plasma concentrations at around 20 and 30 min, respectively. -

Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix
antioxidants Article Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion Marcella Denaro †, Antonella Smeriglio *,† and Domenico Trombetta Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Viale Palatucci, 98168 Messina, Italy; [email protected] (M.D.); [email protected] (D.T.) * Correspondence: [email protected]; Tel.: +39-090-676-4039 † Both authors contributed equally. Abstract: Recently, several studies have highlighted the role of Citrus flavanones in counteracting oxidative stress and inflammatory response in bowel diseases. The aim of study was to identify the most promising Citrus flavanones by a preliminary antioxidant and anti-inflammatory screening by in vitro cell-free assays, and then to mix the most powerful ones in equimolar ratio in order to investigate a potential synergistic activity. The obtained flavanones mix (FM) was then subjected to in vitro simulated digestion to evaluate the availability of the parent compounds at the intestinal level. Finally, the anti-inflammatory activity was investigated on a Caco-2 cell-based model stimulated with interleukin (IL)-1β. FM showed stronger antioxidant and anti-inflammatory activity with respect to the single flavanones, demonstrating the occurrence of synergistic activity. The LC-DAD-ESI- MS/MS analysis of gastric and duodenal digested FM (DFM) showed that all compounds remained unchanged at the end of digestion. As proof, a superimposable behavior was observed between FM and DFM in the anti-inflammatory assay carried out on Caco-2 cells. Indeed, it was observed that both FM and DFM decreased the IL-6, IL-8, and nitric oxide (NO) release similarly to the reference Citation: Denaro, M.; Smeriglio, A.; anti-inflammatory drug dexamethasone. -

Isolation, Identification and Evaluation of Natural Antioxidants from Aromatic Herbs Cultivated in Lithuania
ISOLATION, IDENTIFICATION AND EVALUATION OF NATURAL ANTIOXIDANTS FROM AROMATIC HERBS CULTIVATED IN LITHUANIA CENTRALE LANDBOUWCATALOGUS 0000 0889 7700 Promotor Prof.Dr .Ae . deGroot , hoogleraar ind ebio-organisch e chemie, Wageningen Universiteit Co-promotoren Dr. T.A. vanBeek , universitair hoofddocent, Laboratoriumvoo r Organische Chemie, Wageningen Universiteit Dr. Ir.J.P.H . Linssen, universitair hoofddocent, Sectie Levensmiddelenleer, Wageningen Universiteit Promotiecommissie Prof.Dr .Ir .I.M.C.M . Rietjens (Wageningen Universiteit) Prof.Dr .Ir .P.R .Venskutoni s (Kaunas University ofTechnology , Lithuania) Prof. Dr.Ir .A.G.J . Voragen (Wageningen Universiteit) Dr. H.A.G. Niederlander (Rijksuniversiteit Groningen) /Jf j^30l Airidas Dapkevicius ISOLATION, IDENTIFICATION AND EVALUATION OF NATURAL ANTIOXIDANTS FROM AROMATIC HERBS CULTIVATED IN LITHUANIA (with a summary in English) (met een samenvatting in het Nederlands) (su santrauka lietuviskai) (com resumo em Portugues) Proefschrift ter verkrijging van de graad van doctor op gezagva n de rector magnificus van Wageningen Universiteit, prof. dr. ir. L. Speelman in het openbaar te verdedigen op dinsdag 29januar i 2002 des namiddags te twee uur in de Aula (, 3 Qq I 6 ISBN 90-5808-578-3 Tom y children leva and Simonas General introduction 1.1 Lipid oxidation in general 1 1.2 Food lipid oxidation mechanisms 2 1.2.1 Free radical autoxidation 2 1.2.2 Photooxidation 5 1.2.3 Enzyme-initiated lipid oxidation 6 1.2.4 Metal-catalysed lipid oxidation 6 1.2.5 Formation of secondary products of -

Effect of Ethylene Treatments on Limonin Reduction in Thai Pummelo (Citrus Grandis (L.) Osbeck) Fruit
Kasetsart J. (Nat. Sci.) 45 : 1105 - 1114 (2011) Effect of Ethylene Treatments on Limonin Reduction in Thai Pummelo (Citrus grandis (L.) Osbeck) Fruit Suwanna Pichaiyongvongdee and Ratiporn Haruenkit* ABSTRACT Bitterness in the peel and juice of pummelo is mainly caused by limonin. This research studied different preparations of pummelo and used ethylene as fumigant at different concentrations in different treatments—namely, whole fruit (treatment1), pummelo fruit without the fl avedo and albedo (treatment 2), pummelo fruit with only juice vesicle tissue (treatment 3) and pummelo juice (treatment 4). The limonin content in the fl avedo, albedo, juice vesicle tissues and pummelo juice was signifi cantly reduced following ethylene treatment. For effective limonin reduction in juice, the fl avedo and albedo of pummelo fruit must be removed leaving only juice vesicle tissue (treatment 3) before treating with ethylene. The optimum treatment that reduced the limonin content by 78.38% was 200 ppm ethylene and 1.30 hr exposure. Treating pummelo with ethylene had no effect on nomilin, eriocitrin, neoeriocitrin and the antioxidant capacity of the juice; however, ethylene fumigation caused a slight decrease in the naringin content. Treating pummelo juice directly with ethylene had no effect on its limonin content. Keywords: pummelo, limonin, ethylene INTRODUCTION Pummelo juice is a good source of ascorbic acid with a range from 37.03 to 57.59 mg/100 mL In Thailand, pummelo (Citrus grandis (Pichaiyongvongdee and Haruenkit, 2009a). (L.) Osbeck) is widely grown with many cultivars However, the fruit juice also contains bitter including Kao Yai, Kao Paen, Kao Nampheung, substances such as limonoid with a range from Kao Tanggkya, Kao Hom, Kao Phuang, Pattavee, 20.97 to 67.35 mg/L and fl avanones (naringin, Thongdee and Tha Khoi among others. -

Characterization of a Flavonoid 3'/5'/7-O-Methyltransferase
molecules Article Characterization of a Flavonoid 3’/5’/7-O-Methyltransferase from Citrus reticulata and Evaluation of the In Vitro Cytotoxicity of Its Methylated Products 1,2,3, 1,2,3, 1,2,3 1,2,3 1,2,3 Xiaojuan Liu y, Yue Wang y , Yezhi Chen , Shuting Xu , Qin Gong , Chenning Zhao 1,2,3, Jinping Cao 1,2,3 and Chongde Sun 1,2,3,* 1 College of Agriculture & Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; [email protected] (X.L.); [email protected] (Y.W.); [email protected] (Y.C.); [email protected] (S.X.); [email protected] (Q.G.); [email protected] (C.Z.); [email protected] (J.C.) 2 Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China 3 The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China * Correspondence: [email protected]; Tel.: +86-0571-8898-2229 These authors contributed equally to this work. y Received: 18 January 2020; Accepted: 12 February 2020; Published: 15 February 2020 Abstract: O-methylation of flavonoids is an important modification reaction that occurs in plants. O-methylation contributes to the structural diversity of flavonoids, which have several biological and pharmacological functions. In this study, an O-methyltransferase gene (CrOMT2) was isolated from the fruit peel of Citrus reticulata, which encoding a multifunctional O-methyltransferase and could effectively catalyze the methylation of 3’-, 5’-, and 7-OH of flavonoids with vicinal hydroxyl substitutions. -

Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges
molecules Article Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck) Shengyu Liu 1, Linping Hu 1, Dong Jiang 2 and Wanpeng Xi 1,3,* 1 College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400716, China; [email protected] (S.L.); [email protected] (L.H.) 2 Citrus Research Institute, Chinese Academy of Agricultural Sciences, Chongqing 400712, China; [email protected] 3 Key Laboratory of Horticulture Science for Southern Mountainous Regions, Ministry of Education, Chongqing 400715, China * Correspondence: [email protected]; Tel.: +86-23-68250483; Fax: +86-23-68251274 Received: 20 March 2019; Accepted: 4 May 2019; Published: 6 May 2019 Abstract: To investigate the effect of post-harvest light irradiation on the accumulation of flavonoids and limonoids, harvested Newhall navel oranges were continuously exposed to light-emitting diode (LED) and ultraviolet (UV) light irradiation for 6 days, and the composition and content of flavonoids and limonoids in the segments were determined using UPLC-qTOF-MS at 0, 6, and 15 days after harvest. In total, six polymethoxylated flavonoids (PMFs), five flavone-O/C-glycosides, seven flavanone-O-glycosides, and three limonoids were identified in the segments. The accumulation of these components was altered by light irradiation. Red and blue light resulted in higher levels of PMFs during exposure periods. The accumulation of PMFs was also significantly induced after white light, UVB and UVC irradiation were removed. Red and UVC irradiation induced the accumulation of flavone and flavanone glycosides throughout the entire experimental period. -

Isolation of Eriocitrin (Eriodictyol 7‐O-Rutinoside) from Orange (Citrus Sinuses) Peel by Using Reverse Phase HPLC
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2015): 78.96 | Impact Factor (2015): 6.391 Isolation of Eriocitrin (eriodictyol 7‐O-rutinoside) from Orange (citrus sinuses) Peel by Using Reverse Phase HPLC Syeda Mahejabeen1, P. Nirmala2 1Research Scholar,Department of Biotechnology, Nehru Arts and Science College, Coimbatore, Tamilnadu, India 2Associate Professor, Department of Biotechnology, Nehru Arts and Science College, Coimbatore, Tamilnadu, India Abstract: Orange (Citrus sinuses) is well known for its nutritional and medicinal properties through the world. Citrus belongs to family Rutaceae. In search for anticancer drugs compelling data from laboratories, epidemiologic investigations, and human clinical trials showed that flavonoids have important effects on cancer chemoprevention and chemotherapy. An objective of the present study is to isolate Eriocitrin a flavanone from Orange peel by using Column Chromatography Technique. Orange peel was shadow dried for 7- 10 days and defatted using nHexane, then the residue was extracted using Ethyl Acetate at RT overnight. The extract was air dried and suspended in 1ml of ethyl acetate. A silica gel column of size 300mm X10mm was packed and the 1ml of ethyl acetate extract was loaded onto the column and eluted by using 25 ml ethyl acetate, each 5ml fractions. All these fractions were pooled and checked for Eriocitrin content by HPLC analysis. Results showed that Concentration of Eriocitrin in orange peel powder was found to be 31.57µg/ml. Purity of Eriocitrin by HPLC in Ethyl acetate fraction was found 52 %. Keywords: Eriocitrin, Column Chromatography, RP-HPLC Analysis, UV absorption at 280nms 1. Introduction concentrations 25, 50 and 100 µg/mL were prepared from stock solution. -
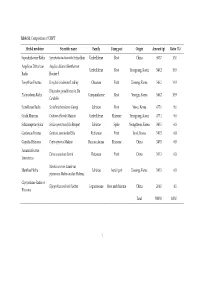
Ratio (%) Saposhnikoviae Ra
Table S1. Composition of CSBPT Herbal medicine Scientific name Family Using part Origin Amount (g) Ratio (%) Saposhnikoviae Radix Saposhnikovia divaricate Schischkin Umbelliferae Root China 500.7 10.0 Angelicae Dahuricae Angelica dahurica Bentham et Umbelliferae Root Yeongyang, Korea 546.2 10.9 Radix Hooker F. Forsythiae Fructus Forsythia viridissima Lindley Oleaceae Fruit Uiseong, Korea 546.2 10.9 Platycodon grandiflorum A. De Platycodonis Radix Campanulaceae Root Yeongju, Korea 546.2 10.9 Candolle Scutellariae Radix Scutellaria baicalensis Georgi Labiatae Root Yeosu, Korea 477.1 9.6 Cnidii Rhizoma Cnidium officinale Makino Umbelliferae Rhizome Yeongyang, Korea 477.1 9.6 Schizonepetae Spica Schizonepeta tenuifolia Briquet Labiatae Spike Yeongcheon, Korea 340.5 6.8 Gardeniae Fructus Gardenia jasminoides Ellis Rubiaceae Fruit Imsil, Korea 340.5 6.8 Coptidis Rhizoma Coptis japonica Makino Ranunculaceae Rhizome China 340.5 6.8 Aurantii Fructus Citrus aurantium Linné Rutaceae Fruit China 340.5 6.8 Immaturus Mentha arvensis Linné var. Menthae Herba Labiatae Aerial part Uiseong, Korea 340.5 6.8 piperascens Malinvaud ex Holmes Glycyrrhizae Radix et Glycyrrhiza uralensis Fischer Leguminosae Root and rhizome China 204.0 4.1 Rhizoma Total 5000.0 100.0 1 Table 2. Chromatographic conditions for simultaneous quantification of compounds 1–18 in CSBPT. Chromatographic parameter Column SunFire C18 analytical column (250 × 4.6 mm, 5 μm) Detector PDA (235, 250, 280, 310, and 345 nm) Flow rate (mL/min) 1.0 Injection volume (μL) 10.0 Column temperature (°C) 40.0 A: 0.1% (v/v) aqueous formic acid Mobile phase B: 0.1% (v/v) formic acid in acetonitrile Time (min) A (%) B (%) 0 95 5 40 40 60 Gradient elution 50 5 95 55 5 95 60 95 5 70 95 5 2 Table S3. -

Flavonoid-Rich Orange Juice Is Associated with Acute
Eur J Nutr (2016) 55:2021–2029 DOI 10.1007/s00394-015-1016-9 ORIGINAL CONTRIBUTION Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males Mudi H. Alharbi2 · Daniel J. Lamport1 · Georgina F. Dodd1 · Caroline Saunders3 · Laura Harkness3 · Laurie T. Butler1 · Jeremy P. E. Spencer2 Received: 26 February 2015 / Accepted: 5 August 2015 / Published online: 18 August 2015 © The Author(s) 2015. This article is published with open access at Springerlink.com Abstract speed was significantly better following the FR drink com- Purpose Epidemiological evidence suggests that chronic pared to the placebo. The effects of objective cognitive consumption of fruit-based flavonoids is associated with function were supported by significant benefits for subjec- cognitive benefits; however, the acute effects of flavonoid- tive alertness following the FR drink relative to the placebo. rich (FR) drinks on cognitive function in the immediate Conclusions These data demonstrate that consumption of postprandial period require examination. The objective was FR orange juice can acutely enhance objective and subjec- to investigate whether consumption of FR orange juice is tive cognition over the course of 6 h in healthy middle-aged associated with acute cognitive benefits over 6 h in healthy adults. middle-aged adults. Methods Males aged 30–65 consumed a 240-ml FR Keywords Flavonoids · Flavanones · Cognition · orange juice (272 mg) and a calorie-matched placebo in a Cognitive function · Orange juice randomized, double-blind, counterbalanced order on 2 days separated by a 2-week washout. Cognitive function and subjective mood were assessed at baseline (prior to drink Introduction consumption) and 2 and 6 h post consumption.