Extraction of Flavanones from Immature Citrus Unshiu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"Performance of Citrus Scion Cultivars and Rootstocks in a High-Density
REPORTS HORTSCIENCE 26(7):837-840. 1991. house and planted in the field in 1981. A split plot experiment and analysis of variance Performance of Citrus Scion Cultivars (ANOVA) statistics were used with four rep- lications, with cultivar as the main plot and and Rootstock in a High-density rootstock as the subplot. Field plots were four ´ four trees, with data taken from the Planting center four of the 16 trees. They were planted 1.5 m in the row and 3.3 m between rows T.A. Wheaton, W.S. Castle, J.D. Whitney, and D.P.H. Tucker and were irrigated and fertigated as required Citrus Research and Education Center, University of Florida, Institute of to maintain optimal soil water and nutrient levels using one microsprinkler per two trees. Food and Agricultural Sciences, 700 Experiment Station Road, Lake Trees were mechanically hedged and topped Alfred, FL 33850 during Summer 1987 and hedged again in 1989 to maintain a 1.5-m alley between rows Additional index words. tree spacing, yield efficiency and a 2.5-m tree height. Thus, the canopy Abstract. ‘Hamlin’ and ‘Valencia’ oranges [Citrus sinensis (L.) Osb.], ‘Murcott’ tangor size allocated for each tree was 1.5 m in the (C. reticulata Blanco ´ C. sinensis), and ‘Redblush’ grapefruit (C. paradisi Macf.) on row, 1.8 m across the row, and 2.5 m in 15 rootstock and own-rooted cuttings were planted at a 1.5 ´ 3.3-m spacing providing height, providing 6.8 m3 of canopy volume a density of 2020 trees/ha. -

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang -

Marketing Standards for Citrus Fruits
COMMISSION IMPLEMENTING REGULATION (EU) No 543/2011 of 7 June 2011 laying down detailed rules for the application of Council Regulation (EC) No 1234/2007 in respect of the fruit and vegetables and processed fruit and vegetables sectors. CONSOLIDATED TEXT: Annex I; Part 2 of Part B MARKETING STANDARD FOR CITRUS FRUIT I. DEFINITION OF PRODUCE This standard applies to citrus fruit of varieties (cultivars) grown from the following species, to be supplied fresh to the consumer, citrus fruit for industrial processing being excluded: - lemons grown from the species Citrus limon (L.) Burm. f. and hybrids thereof, - mandarins grown from the species Citrus reticulata Blanco, including satsumas (Citrus unshiu Marcow), clementines (Citrus clementina hort. ex Tanaka), common mandarins (Citrus deliciosa Ten.) and tangerines (Citrus tangerina Tanaka) grown from these species and hybrids thereof, - oranges grown from the species Citrus sinensis (L.) Osbeck and hybrids thereof. II. PROVISIONS CONCERNING QUALITY The purpose of the standard is to define the quality requirements for citrus fruit after preparation and packaging. However, at stages following dispatch products may show in relation to the requirements of the standard: - a slight lack of freshness and turgidity, - for products graded in classes other than the “Extra” Class, a slight deterioration due to their development and their tendency to perish. A. Minimum requirements In all classes, subject to the special provisions for each class and the tolerances allowed, the citrus fruit must be: - intact, - free of bruising and/or extensive healed overcuts, CI.1 - sound; produce affected by rotting or deterioration such as to make it unfit for consumption is excluded, - clean, practically free of any visible foreign matter, - practically free from pests, - free from damage caused by pests affecting the flesh, - free of signs of shrivelling and dehydration, - free of damage caused by low temperature or frost, - free of abnormal external moisture, - free of any foreign smell and/or taste. -

Glycosides in Lemon Fruit
Food Sci. Technol. Int. Tokyo, 4 (1), 48-53, 1998 Characteristics of Antioxidative Flavonoid Glycosides in Lemon Fruit Yoshiaki MIYAKE,1 Kanefumi YAMAMOT0,1 Yasujiro MORIMITSU2 and Toshihiko OSAWA2 * Central Research Laboratory of Pokka Corporation, Ltd., 45-2 Kumanosyo, Shikatsu-cho, Nishikasugai-gun, Aichi 481, Japan 2Department of Applied Biological Sciences, Nagoya University, Nagoya 46401, Japan Received June 12, 1997; Accepted September 27, 1997 We investigated the antioxidative flavonoid glycosides in the peel extract of lemon fruit (Citrus limon). Six flavanon glycosides: eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, and neohesperidin, and three flavone glycosides: diosmin, 6~-di- C-p-glucosyldiosmin (DGD), and 6- C-p-glucosyldiosmin (GD) were identified by high- performance liquid chromatography (HPLC) analysis. Their antioxidative activity was examined using a linoleic acid autoxidation system. The antioxidative activity of eriocitrin, neoeriocitrin and DGD was stronger than that of the others. Flavonoid glycosides were present primarily in the peel of lemon fruit. There was only a small difference in the content of the flavonoid glycosides of the lemon fruit juice from various sources and varieties. Lemon fruit contained abundant amounts of eriocitrin and hesperidin and also contained narirutin, diosmin, and DGD, but GD, neoeriocitrin, naringin, and neohesperidin were present only in trace amounts. The content of DGD, GD, and eriocitrin was especially abundant in lemons and limes; however, they were scarcely found in other citrus fruits. The content of flavonoid compounds in lemon juice obtained by an in-line extractor at a juice factory was more abundant than that obtained by hand-squeezing. These compounds were found to be stable even under heat treatment conditions (121'C, 15 min) in acidic solution. -

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Artocarpus heterophyllus Lam.: jackfruit, jack, jaca, árbol del pan, jaqueiro X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang cheng, yuzu X Citrus kabuchi hort. ex Tanaka: this is not a published name; could they mean Citrus kinokuni hort. ex Tanaka, kishu mikan? X Citrus limon (L.) Burm. -

High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods
foods Review High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods Mayra Anticona, Jesus Blesa , Ana Frigola and Maria Jose Esteve * Nutrition and Food Chemistry, University of Valencia, Avda., Vicent Andrés Estellés, s/n., 46100 Burjassot, Spain; [email protected] (M.A.); [email protected] (J.B.); [email protected] (A.F.) * Correspondence: [email protected]; Tel.: +34-963544913 Received: 27 April 2020; Accepted: 15 June 2020; Published: 20 June 2020 Abstract: Citrus fruits are extensively grown and much consumed around the world. Eighteen percent of total citrus cultivars are destined for industrial processes, and as a consequence, large amounts of waste are generated. Citrus waste is a potential source of high biological value compounds, which can be used in the food, pharmaceutical, and cosmetic industries but whose final disposal may pose a problem due to economic and environmental factors. At the same time, the emerging need to reduce the environmental impact of citrus waste and its responsible management has increased. For these reasons, the study of the use of non-conventional methods to extract high biological value compounds such as carotenoids, polyphenols, essential oils, and pectins from this type of waste has become more urgent in recent years. In this review, the effectiveness of technologies such as ultrasound assisted extraction, microwave assisted extraction, supercritical fluid extraction, pressurized water extraction, pulsed electric field, high-voltage electric discharges, and high hydrostatic pressures is described and assessed. A wide range of information concerning the principal non-conventional methods employed to obtain high-biological-value compounds from citrus waste as well as the most influencing factors about each technology are considered. -

Absorption and Pharmacokinetics of Grapefruit Flavanones in Beagles
Downloaded from British Journal of Nutrition (2007), 98, 86–92 doi: 10.1017/S0007114507707262 q The Authors 2007 https://www.cambridge.org/core Absorption and pharmacokinetics of grapefruit flavanones in beagles Maria de Lourdes Mata-Bilbao1, Cristina Andre´s-Lacueva1, Elena Roura1, Olga Ja´uregui2, Elvira Escribano3, 4 1 Celina Torre and Rosa Maria Lamuela-Ravento´s * . IP address: 1Department of Nutrition and Food Science, CerTA, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, Barcelona, Spain 2 Scientific and Technical Services, University of Barcelona, Barcelona, Spain 170.106.35.229 3Biopharmaceutics and Pharmacokinetics Unit, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, Barcelona, Spain 4Affinity Pet-care, Barcelona, Spain , on (Received 25 August 2006 – Revised 8 January 2007 – Accepted 24 January 2007) 29 Sep 2021 at 02:36:36 The present study evaluated the pharmacokinetics of three different grapefruit flavanone forms in dog plasma and demonstrated their absorption after an oral intake of a grapefruit extract; pharmacokinetic parameters of these forms were also determined. Ten healthy beagles were adminis- tered 70 mg citrus flavonoids as a grapefruit extract contained in capsules, while two additional dogs were used as controls and given an excipient. The grapefruit flavanone naringin, along with its metabolites naringenin and naringenin glucuronide, was detected in dog plasma. Blood , subject to the Cambridge Core terms of use, available at samples were collected between 0 and 24 h after administration of the extract. Naringin reached its maximun plasma concentration at around 80 min, whereas naringenin and naringenin glucuronide reached their maximun plasma concentrations at around 20 and 30 min, respectively. -

The Satsuma Mandarin1 Peter C
HS195 The Satsuma Mandarin1 Peter C. Andersen and James J. Ferguson2 Scientific Name Family Citrus unshiu Marcovitch Rutaceae Common Name Origin In most citrus producing areas, satsuma mandarin is the China and Japan preferred name, but satsuma tangerine is also used (Figures 1 and 2). Distribution Satsumas are grown in cool subtropical regions of Japan, Spain, central China, Korea, Turkey, along the Black Sea in Russia, southern South Africa, South America, and on a small scale in central California and northern Florida. The world’s largest satsuma industry is located in southern Ja- pan where climatic conditions are favorable for the produc- tion of early ripening satsuma mandarins of high quality. Selection of slight mutations and seedlings from controlled pollinations over many years has resulted in a collection of Figure 1. A satsuma orchard in north Florida. over 100 cultivars that differ in date of maturity, fruit shape, Credits: P. C. Andersen color, and quality. ‘Owari’ is the primary satsuma cultivar commercially grown in Florida, but ‘Brown Select’, ‘Early St. Ann’, ‘Silverhill’, and ‘Kimbrough’ are also available. History Satsuma mandarin may have originated in China, but it was first reported in Japan more than 700 years ago, where it is now the major citrus species grown. The first recorded introduction into the United States was in Florida by George R. Hall in 1876. The name “satsuma” is credited to Figure 2. Mature satsumas ready for harvest. the wife of a United States minister to Japan, General Van Credits: P. C. Andersen 1. This document is HS195, one of a series of the Horticultural Sciences Department, UF/IFAS Extension. -

A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice
molecules Communication A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice Santina Chiechio 1,2,* , Magda Zammataro 1, Massimo Barresi 1, Margherita Amenta 3 , Gabriele Ballistreri 3 , Simona Fabroni 3 and Paolo Rapisarda 3 1 Section of Pharmacology, Department of Drug and Health Sciences, University of Catania, 95123 Catania, Italy; [email protected] (M.Z.); [email protected] (M.B.) 2 Oasi Research Institute IRCCS, 94018 Troina, Italy 3 Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria, Centro di Ricerca Olivicoltura, Frutticoltura e Agrumicoltura, 95024 Acireale, Italy; [email protected] (M.A.); [email protected] (G.B.); [email protected] (S.F.); [email protected] (P.R.) * Correspondence: [email protected] Abstract: Citrus fruits are a rich source of high-value bioactive compounds and their consumption has been associated with beneficial effects on human health. Red (blood) oranges (Citrus sinensis L. Osbeck) are particularly rich in anthocyanins (95% of which are represented by cyanidin-3- glucoside and cyanidin-3-6”-malonyl-glucoside), flavanones (hesperidin, narirutin, and didymin), Citation: Chiechio, S.; Zammataro, and hydroxycinnamic acids (caffeic acid, coumaric acid, sinapic, and ferulic acid). Lemon fruit (Citrus M.; Barresi, M.; Amenta, M.; limon) is also rich in flavanones (eriocitrin, hesperidin, and diosmin) and other polyphenols. All Ballistreri, G.; Fabroni, S.; Rapisarda, of these compounds are believed to play a very important role as dietary antioxidants due to their P. A Standardized Extract Prepared ability to scavenge free radicals. -

Citrus Unshiu Extract
ORYZA OIL & FAT CHEMICAL CO., LTD. CITRUS UNSHIU EXTRACT Health Ingredient for prevention of osteoporosis Health Ingredient for whitening and aesthetic Ingredient for cosmetics CITRUS UNSHIU EXTRACT-P (Powder, Food Grade) CITRUS UNSHIU EXTRACT-L (Liquid, Food Grade) CITRUS UNSHIU EXTRACT-PC (Powder, Cosmetic Grade) CITRUS UNSHIU EXTRACT-LC (Liquid, Cosmetic Grade) ORYZA OIL&FAT CHEMICAL CO., LTD. Ver.3.0HS ver. 2.0SJ CITRUS UNSHIU EXTRACT ver.3.0HS CITRUS UNSHIU EXTRACT Health Ingredient for the prevention of osteoporosis Health Ingredient for whitening and aesthetic Ingredient for cosmetics 1. Introduction Advancement in science & technology and higher standard of living have significantly increased life expectancy in human. Japan is the nation with the highest longevity in the world. However, incidence of osteoporosis increases with increasing life expectancy among the aged population. Osteoporosis is a major and growing public health concern especially for older women and men. Incidence of post-menopausal osteoporosis is high in women aged 50 and above and is classified as primary osteoporosis. More than 90% post-menopausal women are diagnosed with osteoporosis. Risk for dementia increases as complications of osteoporosis related bed-ridden condition Osteoporosis, or porous bone, is a disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures of the hip, spine and wrist. Men as well as women suffer from osteoporosis, a disease that can be prevented and treated. Osteoporosis is a multifactorial disease and is influenced by factors like nutritions, hormone and physical activity. The process of bone resorption and reformation is continuous and osteoclasts are important in the regulation of bone metabolism. -

Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges
molecules Article Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck) Shengyu Liu 1, Linping Hu 1, Dong Jiang 2 and Wanpeng Xi 1,3,* 1 College of Horticulture and Landscape Architecture, Southwest University, Chongqing 400716, China; [email protected] (S.L.); [email protected] (L.H.) 2 Citrus Research Institute, Chinese Academy of Agricultural Sciences, Chongqing 400712, China; [email protected] 3 Key Laboratory of Horticulture Science for Southern Mountainous Regions, Ministry of Education, Chongqing 400715, China * Correspondence: [email protected]; Tel.: +86-23-68250483; Fax: +86-23-68251274 Received: 20 March 2019; Accepted: 4 May 2019; Published: 6 May 2019 Abstract: To investigate the effect of post-harvest light irradiation on the accumulation of flavonoids and limonoids, harvested Newhall navel oranges were continuously exposed to light-emitting diode (LED) and ultraviolet (UV) light irradiation for 6 days, and the composition and content of flavonoids and limonoids in the segments were determined using UPLC-qTOF-MS at 0, 6, and 15 days after harvest. In total, six polymethoxylated flavonoids (PMFs), five flavone-O/C-glycosides, seven flavanone-O-glycosides, and three limonoids were identified in the segments. The accumulation of these components was altered by light irradiation. Red and blue light resulted in higher levels of PMFs during exposure periods. The accumulation of PMFs was also significantly induced after white light, UVB and UVC irradiation were removed. Red and UVC irradiation induced the accumulation of flavone and flavanone glycosides throughout the entire experimental period. -
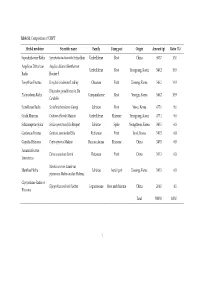
Ratio (%) Saposhnikoviae Ra
Table S1. Composition of CSBPT Herbal medicine Scientific name Family Using part Origin Amount (g) Ratio (%) Saposhnikoviae Radix Saposhnikovia divaricate Schischkin Umbelliferae Root China 500.7 10.0 Angelicae Dahuricae Angelica dahurica Bentham et Umbelliferae Root Yeongyang, Korea 546.2 10.9 Radix Hooker F. Forsythiae Fructus Forsythia viridissima Lindley Oleaceae Fruit Uiseong, Korea 546.2 10.9 Platycodon grandiflorum A. De Platycodonis Radix Campanulaceae Root Yeongju, Korea 546.2 10.9 Candolle Scutellariae Radix Scutellaria baicalensis Georgi Labiatae Root Yeosu, Korea 477.1 9.6 Cnidii Rhizoma Cnidium officinale Makino Umbelliferae Rhizome Yeongyang, Korea 477.1 9.6 Schizonepetae Spica Schizonepeta tenuifolia Briquet Labiatae Spike Yeongcheon, Korea 340.5 6.8 Gardeniae Fructus Gardenia jasminoides Ellis Rubiaceae Fruit Imsil, Korea 340.5 6.8 Coptidis Rhizoma Coptis japonica Makino Ranunculaceae Rhizome China 340.5 6.8 Aurantii Fructus Citrus aurantium Linné Rutaceae Fruit China 340.5 6.8 Immaturus Mentha arvensis Linné var. Menthae Herba Labiatae Aerial part Uiseong, Korea 340.5 6.8 piperascens Malinvaud ex Holmes Glycyrrhizae Radix et Glycyrrhiza uralensis Fischer Leguminosae Root and rhizome China 204.0 4.1 Rhizoma Total 5000.0 100.0 1 Table 2. Chromatographic conditions for simultaneous quantification of compounds 1–18 in CSBPT. Chromatographic parameter Column SunFire C18 analytical column (250 × 4.6 mm, 5 μm) Detector PDA (235, 250, 280, 310, and 345 nm) Flow rate (mL/min) 1.0 Injection volume (μL) 10.0 Column temperature (°C) 40.0 A: 0.1% (v/v) aqueous formic acid Mobile phase B: 0.1% (v/v) formic acid in acetonitrile Time (min) A (%) B (%) 0 95 5 40 40 60 Gradient elution 50 5 95 55 5 95 60 95 5 70 95 5 2 Table S3.