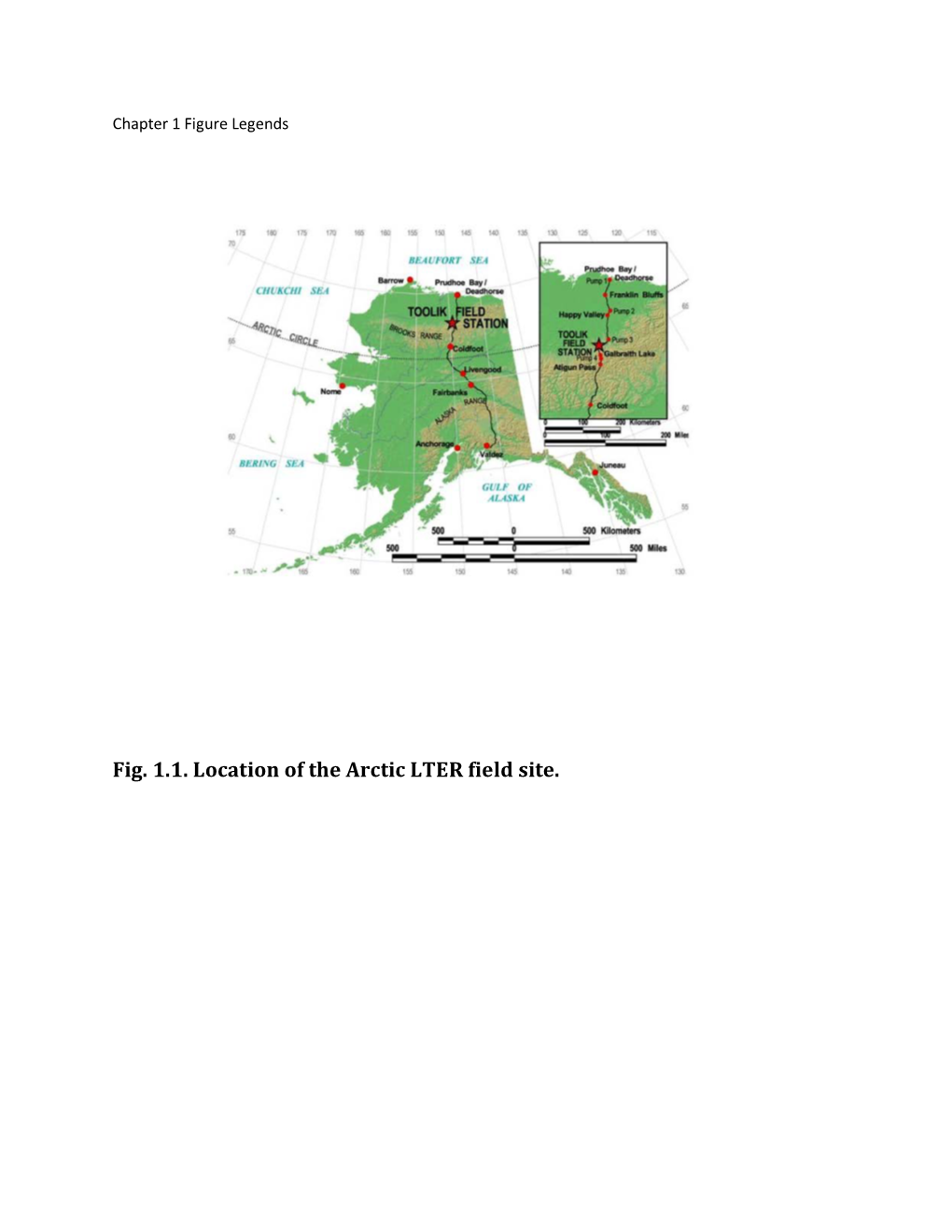
Fig. 1.1. Location of the Arctic LTER Field Site
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Changing Arctic: Ecological Consequences for Tundra, Streams and Lakes
A CHANGING ARCTIC: ECOLOGICAL CONSEQUENCES FOR TUNDRA, STREAMS AND LAKES Edited by John E. Hobbie George W. Kling Chapter 1. Introduction Chapter 2. Climate and Hydrometeorology of the Toolik Lake Region and the Kuparuk River Basin: Past, Present, and Future Chapter 3. Glacial History and Long-Term Ecology of the Toolik Lake Region Chapter 4. Late-Quaternary Environmental and Ecological History of the Arctic Foothills, Northern Alaska Chapter 5. Terrestrial Ecosystems Chapter 6. Land-Water Interactions Research Chapter 7. Ecology of Streams of the Toolik Region Chapter 8. The Response of Arctic-LTER Lakes to Environmental Change Chapter 9. Mercury in the Alaskan Arctic Chapter 10. Ecological consequences of present and future change 1 <1>Chapter 1. Introduction John E. Hobbie <1>Description of the Arctic LTER site and project Toolik, the field site of the Arctic Long Term Ecological Research (LTER) project, lies 170 km south of Prudhoe Bay in the foothills of Alaska’s North Slope near the Toolik Field Station (TFS) of the University of Alaska Fairbanks (Fig. 1.1).[INSERT FIGURE 1.1 HERE] The project goal is to describe the communities of organisms and their ecology, to measure changes that are occurring, and to predict the ecology of this region in the next century. Research at the Toolik Lake site began in the summer of 1975 when the completion of the gravel road alongside the Trans-Alaska Pipeline, now called the Dalton Highway, opened the road-less North Slope for research. This book synthesizes the research results from this site since 1975, as supported by various government agencies but mainly by the U.S. -

Dalton Corridor Region
Chapter 3: Dalton Corridor Region Dalton Corridor Region (D) The Dalton Corridor Region encompasses an area of approximately 1.1 million acres, extending from the Chandalar Shelf at the Umiat Meridian, North to the terminus of the Dalton Highway in Deadhorse. The Dalton Corridor Region is defined by the James Dalton Highway, and legislatively identified in AS 19.40 as an area within five miles of the highway right-of-way. North of the Brooks Range the region contains the Sagavanirktok (“Sag”) River on its northerly flow to Deadhorse. The Trans-Alaska Pipeline System (TAPS) generally parallels the Dalton Highway, and also falls within the region. The terrain varies widely across the region from end to end, and transitions from high alpine tundra and rugged mountains at the southern end, to low arctic tundra and wetlands on the northern extent. This region embodies the greater North Slope area and is a uniquely contiguous transect from the peak of the Brooks Range down to the toe of the slope. All lands within the region are either state-owned or state-selected, with the exception of the area between Galbraith Lake and Atigun Pass which are within five miles of Arctic Refuge, and the Chandalar Shelf area which is within five miles of Gates of the Arctic National Park and Preserve. In these areas, the region boundary follows the LDA. North of Happy Valley there are numerous active municipal entitlement selections by the North Slope Borough. Distribution and Characteristics Within the corridor region, approximately 1/3 of the area, encompassing the entire southern portion, is comprised of federal public land managed by BLM that is state-selected or top filed under Section 906(e) of the Alaska National Interest Lands Conservation Act (ANILCA). -

Cryosols and Arctic Tundra Ecosystems, Alaska July 16-22, 2006
WCSS Post-Conference Tour #1 Cryosols and Arctic Tundra Ecosystems, Alaska July 16-22, 2006 Published by the School of Natural Resources & Agricultural Sciences and the Agricultural & Forestry Experiment Station, University of Alaska Fairbanks, with funding from the Agronomy Society of America. UAF is an AA/EO employer and educational institution. Publication #MP 2006-03 e; available on line at www.uaf.edu/snras/afes/pubs/ UAF is an AA/EO employer and educational institution. WCSS Post-Conference Tour #1 Cryosols and Arctic Tundra Ecosystems, Alaska July 16-22, 2006 Chien-Lu Ping, Tour Leader School of Natural Resources and Agricultural Sciences University of Alaska Fairbanks Sponsors University of Alaska Fairbanks USDA-NRCS Alaska State Office USDA-NRCS National Soil Survey Center Soil Science Society of America Contents 2……Itinerary 4……Participants 4……Acknowledgments 5……Tour Information July 16, Sunday July 17, Monday 8……S - 1. Typic Eutrocryept, on south-facing slope, Smith Lake, Fairbanks 11……S - 2. Typic Historthel, on south facing toe slope, Smith Lake, Fairbanks 14……S - 3. Ruptic Histoturbel, under tussocks in valley floor, Smith Lake, Fairbanks 16……S - 4. Fluventic Historthel, north facing slope, Smith Lake, Fairbanks July 18, Tuesday July 19, Wednesday 19……S - 5. Ice wedge deterioration along the Sagavanirktok River, North Slope 21……S - 6. Fluvaquentic Historthel, in low-center polygons, North Slope July 20, Thursday 24……S - 7. Ruptic-Histic Aquiturbel, nonsorted circles under moist nonacidic tundra, Sagwon Hills, North Slope 26……S - 8. Ruptic-Histic Aquiturbel, nonsorted circles in moist acidic tundra, Sagwon Hills, North Slope July 21, Friday 29……S - 9. -

Galbraith Lake Airport and Access Road
Galbraith Lake Airport and Access Road Compliance The proposed action is in conformance with the approved Bureau of Land Management Utility Corridor Resource Management Plan approved January 11, 1991. The project has been considered in the context of public health and safety and consistency with regards to Federal, State, and local laws. Selected Action The proposed action described in the Category Exclusion mentioned below is the selected action. A twenty (20) year airport lease case file F-12632 and a twenty (20) year right-of-way grant case file F-91217 for the access road from the Dalton Highway MP 275 to the airport will be issued to the State of Alaska, Department of Transportation and Public Facilities. This lease and grant is for the continued operation of the Galbraith Lake Airport and the access road to the airport. Compliance with NEPA: The proposed action is categorically excluded from further documentation under the National Environmental Policy Act (NEPA) in accordance with United States Department of the Interior 43 CFR §46.210 or United States Department of Interior Manual, Part 516, Chapter 11 which provides: 11.9 E (Realty) (9) Renewals and assignments of leases, permits, or rights-of-way where no additional rights are conveyed beyond those granted by the original authorizations. Public Involvement: It was determined that no public involvement was needed due to the remoteness of the action Rationale: The proposed action is consistent with the use of public lands under the authorities of Titles III and V of the Federal Land Policy and Management Act and the regulations found in 43 CFR 2920 and 43 CFR 2800. -
Dalton Highway Field Trip Guide for the Ninth International Conference on Permafrost
DALTON HIGHWAY FIELD TRIP GUIDE FOR THE NINTH INTERNATIONAL CONFERENCE ON PERMAFROST A supplement to Guidebook 4, “Guidebook to permafrost and related features along the Elliott and Dalton Highways, Fox to Prudhoe Bay, Alaska,” 1983, by Jerry Brown and R.A. Kreig, editors, published by the Alaska Division of Geological & Geophysical Surveys for the Fourth International Conference on Perma- frost. by D.A. Walker, T.D. Hamilton, C.L. Ping, R.P. Daanen, and W.W. Streever With contributions by M.S. Bret-Harte, R.R. Gieck, T.N. Hollingsworth, L.S. Jodwallis, D.L. Kane, G.J. Michaelson, F.E. Nelson, C.A. Munger, M.K. Raynolds, V.E. Romanovsky, G.R. Shaver, E.M. Barbour, C.A. Stiles Guidebook 9 Published by STATE OF ALASKA DEPARTMENT OF NATURAL RESOURCES DIVISION OF GEOLOGICAL & GEOPHYSICAL SURVEYS 2009 DALTON HIGHWAY FIELD TRIP GUIDE FOR THE NINTH INTERNATIONAL CONFERENCE ON PERMAFROST A supplement to Guidebook 4, “Guidebook to permafrost and related features along the Elliott and Dalton Highways, Fox to Prudhoe Bay, Alaska,” 1983, by Jerry Brown and R.A. Kreig, editors, published by the Alaska Division of Geological & Geophysical Surveys for the Fourth International Conference on Permafrost. by D.A. Walker, T.D. Hamilton, C.L. Ping, R.P. Daanen, and W.W. Streever With contributions by M.S. Bret-Harte, University of Alaska Fairbanks; R.R. Gieck, University of Alaska Fairbanks; T.N. Hollingsworth, U.S. Forest Service, Bonanza Creek Research Unit; L.S. Jodwallis, Bureau of Land Management; D.L. Kane, University of Alaska Fairbanks; G.J. Michaelson, University of Alaska Fairbanks; F.E. -

Decision Record Memorandum Crew/Man Camp at Galbraith Lake
Decision Record Memorandum Crew/Man Camp at Galbraith Lake Introduction Summary This camp would be occupied by BorTek’s general contractor North Slope Telecom Inc. (NSTI) and their sub-contractors. They propose a one (1) year authorization. During the construction phase of work north of Atigun, Bortek is requesting to install a “man- camp” at the Galbraith site. They propose a temporary crew/man camp mobilized to the site for a seasons use, provided by a professional camp supplier/operator. The operator (Black Gold) will provide all supplies, including hauling of wastes. The camp location would also serve as a storage area for construction materials and the parking of vehicles and equipment. Materials to be stored on-site include reels of 1-1/4” HDPE conduit, reels of fiber optic cable, and handholes/manholes made of composite materials. The crew/man camp would include establishment of 2 units. The first is 6 self-contained units joined together to create one building, the second is 4 units joined to create one building. These buildings are composed of sleeping quarters, restrooms, kitchen and dining facilities. There would also be 3 additional support units: an 8’x48’ van for water, an 8’x40’ for sewer, and a genset (generator). All solid waste generated would be stored in a dumpster and hauled as needed to maintain a clean site. All sewage wastes would be hauled by an authorized handler and disposed of properly. Water would be hauled from a commercial source and stored onsite as needed for camp use. The camp would also have a 10,000 gallon double walled fuel tank for the storage of diesel. -

Toolik Lake Research Natural Area / ACEC Rare Plant Inventory, 2002
U. S. Department of the Interior BLM-Alaska Open File Report 90 Bureau of Land Management BLM/AK/ST-03/010+6700+025 July 2003 Alaska State Office 222 West 7th Avenue Anchorage, Alaska 99513 Toolik Lake Research Natural Area / ACEC Rare Plant Inventory, 2002 Amy Breen Carroll, Carolyn Parker, Tim Craig Cover Photo Claytoniella bostockii (A. E. Pors.) Jurtz (photo by M. Tachibana). Authors Amy Breen Carroll works with the Institute of Arctic Biology as well as the Biology and Wildlife Departments at the University of Alaska, Fairbanks. Carolyn Parker is a research associate at the University of Alaska Museum Herbarium in Fairbanks. Tim Craig is a Wildlife Biologist in the BLM-Alaska Northern Field Office. The BLM Mission The Bureau of Land Management sustains the health, diversity and productivity of the public lands for the use and enjoyment of present and future generations. Disclaimer The mention of trade names or commercial products in this report does not constitute endorsement or recom mendation for use by the federal government. Open File Reports Open File Reports issued by the Bureau of Land Management-Alaska present the results of inventories or other investigations on a variety of scientific and technical subjects that are made available to the public outside the formal BLM-Alaska technical publication series. These reports can include preliminary or incomplete data and are not published or distributed in quantity. The reports are available while supplies last from BLM External Affairs, 222 West 7th Avenue #13, Anchorage, Alaska 99513, telephone (907) 271-3318; and from the Juneau Minerals Information Center, 100 Savikko Road, Mayflower Island, Douglas, AK 99824, (907) 364-1553. -

The Trans-Alaska Pipeline 1 2
www.PDHcenter.com www.PDHonline.org River of Oil Table of Contents Slide/s Part Description 1N/ATitle 2 N/A Table of Contents 3~63 1 Running on Empty 64~100 2 Native Wealth 101~191 3 One If By Land 192~266 4 Two If By Sea 267~284 5 Three If By Air 285~346 6 Touch the Earth Gently 347~434 7 Safeguarding the Future 435~514 8 Wild Alaska 515~631 9 JFWAT 632~706 10 Trial & Error 707~804 11 Concern No. 3 805~856 12 The Report Card 857~919 13 Turning On the TAPS 920~950 14 A Never Ending Story The Trans-Alaska Pipeline 1 2 Part 1 Dead Serious Running on Empty 3 4 “‘Anyone who buys who buys one of those big gas-eating cars has got to be out of his mind,’ said the man sitting across the desk from me. For a moment, I couldn’t believe my ears. I’ve heard that kind of talk before, but always from professional car-haters. This time, thougg,h, it was coming from one of the top executives in the oil industry - he doesn’t Trouble Ahead want his name used for obvious reasons - whose livelihood depends on selling lots of gasoline to power lots of cars. But he was dead serious, and he has lots of company…” Popular Science, April 1973 5 6 © J.M. Syken 1 www.PDHcenter.com www.PDHonline.org “…In several weeks of interviewing experts on energy in general and the oil industry in particular, I got nothing but predictions of desperateldesperately hard times ahead for America’s What Kind of Trouble? auto owners. -

Galbraith Lake Airport and Access Road A
Galbraith Lake Airport and Access Road A. Background DOI-BLM-AK-F030–2013–0049CX Case File Nos.: F-12632 and F-91217 Proposed Action Title/Type: Galbraith Lake Airport and Access Road Renewal Location of Proposed Action: Galbraith Lake Airport is located at MP 275 of the Dalton Highway approximately 106 miles south of Prudhoe Bay and 355 miles northwest of Fairbanks, Alaska more particularly described as Secs. 14, 15, 22, 23 and 26, T. 11 S., R. 11 E., Umiat Meridian. USGS Quad Phillip Smith Mountains. Description of Proposed Action: The State of Alaska, Department of Transportation and Public Facilities (SOA/DOTPF) proposes to renew their airport lease and road right-of-way for access to the airport for 20 years. The airport was constructed in 1974–75 to support the construction of the Trans-Alaska Pipeline System and the Dalton Highway. The site, generally unchanged since construction encompasses a 625 acre rectangle. The 625 acres is subdivided into seven (7) parcels described as Parcels A, B, C, D, E and Lots 1 and 2. Parcel A is reserved for BLM use and has a FWS bunkhouse and camp. The airport maintenance is provided by Alyeska Pipeline Service Company through a written agreement with the SOA/DOTPF. Lot 1 has been permitted to the Alaska Fish and Game, Lot 2 permitted to Richard Guthrie for a guiding business and Parcel E to the University of Alaska for research. The runway has a gravel surface measuring 5,200' x 150', and an unpaved apron 400' x 200'. The airport is lighted with medium intensity edge lighting, lighted wind cones, apron floodlighting and runway end identifier lights on both ends of the runway. -

Rare Vascular Plants of the Blm Dalton Highway Utility
RARE VASCULAR PLANTS OF THE BLM DALTON HIGHWAY UTILITY CORRIDOR A report by Robert Lipkin and Carolyn Parker Alaska Natural Heritage Program Environment and Natural Resources Institute University of Alaska Anchorage and University of Alaska Museum Fairbanks August 1995 ACKNOWLEDGEMENTS This report is a continuation of the cooperative relationship begun in 1990 between the Bureau of Land Management and the Alaska Natural Heritage Program. We would especially like to thank Dave Yokel of the Arctic District, BLM, who conceived this particular project, secured the funding for it, and has evinced continual concern for the rare plants of the District. Thanks also go to David Murray, Curator Emeritus, and Alan Batten, Research Associate, of the Herbarium of the University of Alaska Museum for their cooperation and assistance. Their collections, database, and literature files provided the basis for this report. David Murray also provided a valuable review of species rankings and taxonomy for some of the taxa in this report. i TABLE OF CONTENTS Acknowledgments .................................................................................................................................. i Table of Contents................................................................................................................................... ii Introduction Background ................................................................................................................................. 1 Information Sources and Ranking of Rare Plants ...............................................................