Cerebrovascular Diseases
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Management of the Head Injury Patient
Management of the Head Injury Patient William Schecter, MD Epidemilogy • 1.6 million head injury patients in the U.S. annually • 250,000 head injury hospital admissions annually • 60,000 deaths • 70-90,000 permanent disability • Estimated cost: $100 billion per year Causes of Brain Injury • Motor Vehicle Accidents • Falls • Anoxic Encephalopathy • Penetrating Trauma • Air Embolus after blast injury • Ischemia • Intracerebral hemorrhage from Htn/aneurysm • Infection • tumor Brain Injury • Primary Brain Injury • Secondary Brain Injury Primary Brain Injury • Focal Brain Injury – Skull Fracture – Epidural Hematoma – Subdural Hematoma – Subarachnoid Hemorrhage – Intracerebral Hematorma – Cerebral Contusion • Diffuse Axonal Injury Fracture at the Base of the Skull Battle’s Sign • Periorbital Hematoma • Battle’s Sign • CSF Rhinorhea • CSF Otorrhea • Hemotympanum • Possible cranial nerve palsy http://health.allrefer.com/pictures-images/ Fracture of maxillary sinus causing CSF Rhinorrhea battles-sign-behind-the-ear.html Skull Fractures Non-depressed vs Depressed Open vs Closed Linear vs Egg Shell Linear and Depressed Normal Depressed http://www.emedicine.com/med/topic2894.htm Temporal Bone Fracture http://www.vh.org/adult/provider/anatomy/ http://www.bartleby.com/107/illus510.html AnatomicVariants/Cardiovascular/Images0300/0386.html Epidural Hematoma http://www.chestjournal.org/cgi/content/full/122/2/699 http://www.bartleby.com/107/illus769.html Epidural Hematoma • Uncommon (<1% of all head injuries, 10% of post traumatic coma patients) • Located -

Endovascular Management of Acute Epidural Hematomas: Clinical Experience with 80 Cases
CLINICAL ARTICLE J Neurosurg 128:1044–1050, 2018 Endovascular management of acute epidural hematomas: clinical experience with 80 cases Carlos Michel A. Peres, MD,1 Jose Guilherme M. P. Caldas, MD, PhD,2 Paulo Puglia Jr., MD,2 Almir F. de Andrade, MD, PhD,3 Igor A. F. da Silva, MD,3 Manoel J. Teixeira, MD, PhD,3 and Eberval G. Figueiredo, MD, PhD3 1Hospital Universitário Francisca Mendes, Manaus; and Divisions of 2Neuroradiology and 3Neurosurgery, University of São Paulo School of Medicine, São Paulo, Brazil OBJECTIVE Small acute epidural hematomas (EDHs) treated conservatively carry a nonmeasurable risk of late en- largement due to middle meningeal artery (MMA) lesions. Patients with EDHs need to stay hospitalized for several days, with neurological supervision and repeated CT scans. In this study, the authors analyzed the safety and efficacy of the embolization of the involved MMA and associated lesions. METHODS The study group consisted of 80 consecutive patients harboring small- to medium-sized EDHs treated by MMA embolization between January 2010 and December 2014. A literature review cohort was used as a control group. RESULTS The causes of head injury were falls, traffic-related accidents (including car, motorcycle, and pedestrian vs vehicle accidents), and assaults. The EDH topography was mainly temporal (lateral or pole). Active contrast leaking from the MMA was seen in 57.5%; arteriovenous fistulas between the MMA and diploic veins were seen in 10%; and MMA pseudoaneurysms were found in 13.6% of the cases. Embolizations were performed under local anesthesia in 80% of the cases, with N-butyl-2-cyanoacrylate, polyvinyl alcohol particles, or gelatin sponge (or a combination of these), obtaining MMA occlusion and complete resolution of the vascular lesions. -

Spontaneous Cervical Spinal Epidural Hematoma
CASE REPORT Spontaneous Cervical Spinal Epidural Hematoma Katie Rinne, MBBS; Sunil Gopisetty, MRCEM A 68-year-old woman presented with sudden-onset right arm and leg weakness, as well as right-sided neck pain. nilateral weakness is a common ED and was a nonsmoker with a normal body presentation with a diverse etiology, mass index. Her family history was signifi- including stroke.1,2 Studies have re- cant for cerebral vascular accidents. U ported a misdiagnosis rate of stroke On arrival at the ED, the patient had a and transient ischemic attack of approxi- blood pressure of 179/95 mm Hg; her other mately 10%.3 This case presents an un- vital signs were normal. On examination, usual stroke mimic where treatment with she had a right-sided hemiplegia, with a 0/5 an anticoagulant could have led to adverse power grading observed for motor strength outcomes. It also highlights the importance for both her right arm and leg. She reported of considering a spontaneous cervical spi- paresthesia in dermatomes C4 to C5 and L3 to nal epidural hematoma (SCSEH) as a stroke L5. There was extreme tenderness when her mimic. This is especially pertinent when a right trapezius and upper paraspinal muscles patient’s symptoms are not fully consistent were palpated, but she had no midline cer- with an acute stroke, in order to avoid po- vical spine tenderness and had full, though tentially dangerous anticoagulation and al- painful, range of movement of her neck. Her low for prompt treatment of the hematoma. left side was unaffected. She had normal cra- nial nerves, no higher cortical dysfunction, a Case Glasgow Coma Scale (GCS) score of 15, and A 68-year-old woman presented to the ED via complete control of her bladder and bowel. -

Intracranial Hemorrhage
Intracranial Hemorrhage MARK MOSS, M.D. INTERVENTIONAL NEURORADIOLOGY WASHINGTON REGIONAL MEDICAL CENTER Definitions Stroke Clinical syndrome of rapid onset deficits of brain function lasting more than 24 hours or leading to death Transient Ischemic attack (TIA) Clinical syndrome of rapid onset deficits of brain function which resolves within 24 hours Epidemiology Stroke is the leading cause of adult disabilities 2nd leading cause of death worldwide 3rd leading cause of death in the U.S. 800,000 strokes per year resulting in 150,000 deaths Deaths are projected to increase exponentially in the next 30 years owing to the aging population The annual cost of stroke in the U.S. is estimated at $69 billion Stroke can be divided into hemorrhagic and ischemic origins 13% hemorrhagic 87% ischemic Intracranial Hemorrhage Collective term encompassing many different conditions characterized by the extravascular accumulation of blood within different intracranial spaces. OBJECTIVES: Define types of ICH Discuss best imaging modalities Subarachnoid hemorrhage / Aneurysms Roles of endovascular surgery Intracranial hemorrhage Outside the brain (Extra-axial) hemorrhage Subdural hematoma (SDH) Epidural hematoma (EDH) Subarachnoid hematoma (SAH) Intraventricular (IVH) Inside the brain (Intra-axial) hemorrhage Intraparenchymal hematoma (basal ganglia, lobar, pontine etc.) Your heads compartments Scalp Subgaleal Space Bone (calvarium) Dura Mater thick tough membrane Arachnoid flimsy transparent membrane Pia Mater tightly hugs the -

Traumatic Brain Injury in Boxing and Mixed Martial Arts Dr
TRAUMATIC BRAIN INJURY IN BOXING AND MIXED MARTIAL ARTS DR. DOMENIC F COLETTA,JR. CHIEF RINGSIDE PHYSICIAN NEW JERSEY STATE ATHLETIC CONTROL BOARD ACUTE TRAUMATIC BRAIN INJURY (ATBI) • DIFFUSE BRAIN INJURY - Diffuse axonal injury (KO) - Cerebral concussion - Second Impact Syndrome • FOCAL BRAIN INJURY - Subdural and epidural hemorrhage - Cerebral contusion - Other cerebral hemorrhages (rare) COMMON SIGNS AND SYMPTOMS OF ATBI • Cognitive features - Decreased speed of information processing - Confusion/ Disorientation - Amnesia/ Memory impairment - Impaired concentration - Loss of consciousness • Behavioral features - Irritability/ Anxiety - Sleep disturbance - Fatigue/ Apathy/ Psychomotor retardation - Easily distracted • Physical features - Headache/ Dizziness - Nausea - Impaired coordination/ ataxic gait - Visual disturbances - Vacant stare - Seizure PATHOPHYSIOLOGY OF BRAIN INJURY IN BOXING Three types of stresses to the brain. 1. Compressive 2. Stretching (tensile) 3. Shearing – the most dangerous HEAD BLOWS • Rotational (angular) acceleration – blows to the side of the head or to the chin that produce the greatest shearing forces causing direct neuronal and vascular damage, either focal or diffuse. • Translational (linear) acceleration – jabs to the face that are less dangerous but still can account for coup – contrecoup injury to the brain. • Impact deceleration – occurs when head strikes the mat after a KO and may cause an additional traumatic brain injury. CONCUSSION • A complex pathophysiologic process that affects the brain and is induced by traumatic biomechanical forces. • Typically it is a rapid onset of short-lived impairment of neurological function that resolves spontaneously (although a small percentage will have prolonged post- concussion symptoms. • It is difficult to diagnose during a boxing match because most concussions are not associated with LOC and, a fighter who gets KO’d may or may not be concussed. -

Spontaneous Cervical Epidural Hematoma Mimicking Acute Ischemic Stroke
Published Ahead of Print on August 4, 2020 as 10.1212/WNL.0000000000010511 Rahangdale 1 Neurology Publish Ahead of Print DOI: 10.1212/WNL.0000000000010511 Spontaneous cervical epidural hematoma mimicking acute ischemic stroke Rahul Rahangdale, MD1, John Coburn, MD2, Christopher Streib, MD1 1 Department of Neurology, University of Minnesota, Minneapolis MN 2 Division of Neuroradiology, Midwest Radiology PA, St. Paul, MN Corresponding author: Rahul Rahangdale, Email: [email protected] Title character count: 70 Abstract word count: 0 Manuscript word count: 100 Legends word count: 50 References: 2 Figures: 2 Neurology® PublishedACCEPTED Ahead of Print articles have been peer reviewed and accepted for publication. This manuscript will be published in its final form after copyediting, page composition, and review of proofs. Errors that could affect the content may be corrected during these processes. Copyright © 2020 American Academy of Neurology. Unauthorized reproduction of this article is prohibited Rahangdale 2 Keywords: Spontaneous cervical spinal epidural hematoma, Stroke mimics, IV tPA Disclosure: R.Rahangdale. reports no disclosures relevant to the manuscript; J.Coburn. reports no disclosures relevant to the manuscript; C.Streib. reports no disclosures relevant to the manuscript. Study funding: No targeted funding reported. Manuscript: A 67-year-old male presented with acute right hemiparesis and hemianesthesia (NIHSS 5). Hyperacute neuroimaging was interpreted as normal. Following IV tPA, his symptoms worsened with concern for cervical radiculopathy. Upon reviewing the CT angiogram (CTA), a possible spinal epidural hematoma (EDH) was noted and subsequently confirmed with MRI (figure 1). Cryoprecipitate was administered. His symptoms gradually improved with conservative management. Spontaneous spinal EDH is rare (incidence: 0.1/100,000 per year).1 Risk factors include hypertension and 1 ACCEPTED2 coagulopathy. -

Spontaneous Cervical Epidural Hematoma: Case Report
Spinal Cord (1998) 36, 71 ± 72 1998 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/98 $12.00 Spontaneous cervical epidural hematoma: case report Adnan Awada1, Neville Russell2, Naif Al Fayez2, Richard Naufal3 and Hussein Al Kohlani2 Sections of 1Neurology and 2Neurosurgery and 3Department of Medical Imaging, King Fahd National Guard Hospital, POB 22490, Riyadh 11426, Saudi Arabia Spontaneous spinal epidural hematoma (SSEH) is an uncommon cause of acute nontraumatic myelopathy. We report a 14 year-old boy who had tetraplegia on awakening. Diagnosis of spinal epidural hematoma was made by magnetic resonance imaging. Despite spinal cord decompression within 9 h from onset, he remained tetraplegic. No cause for the bleeding was found. The pathogenic hypotheses of SSEH are discussed and the importance of rapid diagnosis and treatment is emphasized. Keywords: spinal cord; epidural hematoma; magnetic resonance imaging (MRI); acute myelopathy; tetraplegia Introduction Spinal epidural hematoma is a very rare cause of acute stimulation. Urinary retention and priapism were spinal cord compression. It is important to recognize it present. An acute cervical spinal cord disorder was since early diagnosis and prompt surgical evacuation suspected and an urgent magnetic resonance imaging provide the maximum chance of functional recovery. (MRI) examination ordered. This was performed at Spinal epidural hematoma has been reported in 3 pm and on T1 and T2-weighted images, revealed an association with blood dyscrasias, coagulopathies, iso-intense mass, centered at the level of C6 (a `double anti-coagulant treatment, infection, tumor, pregnancy cord' image) (Figure 1). This was compressing the and vascular malformations, but approximately half of dorsal aspect of the spinal cord. -

Chapter 2 Concussion Mechanisms And
CHAPTER 2 CONCUSSION MECHANISMS AND PATHOPHYSIOLOGY Jack Wilberger\ Juan Ortega^ & Semyon Slobounov^ ^Chairman, Department of Neurosurgery, Vice Dean Drexel University College of Medicine, Allegheny General Hospital, Pittsburgh, PA. ^Department of Neurosurgery, Allegheny General Hospital, Pittsburgh, PA. ^Department of Kinesiology, The Pennsylvania State University, University Park, PA. Abstract: Concussions are a frequent occurrence in athletic endeavors, its rate exceeding that occurring in the general population by 50 fold. The biomechanics and pathophysiology of concussion are still not well understood and may lead to potential significant sequelae from single or more commonly multiple concussions. Postconcussive symptoms, the second impact syndrome and the cumulative effects of concussions are all topics of interest in current concussion research in athletes and are leading to a more rational approach in determining policy aimed at returning athletes to their sport after a concussion. This chapter reviews current knowledge on the mechanisms, pathophysiology and sequelae of concussion in athletes. Keywords: Concussion; Metabolic cascade; Glucose utilization; Ionic changes; Epidural hematoma; Subdural Hematoma; Intracranial Hemorrhage. 1. INTRODUCTION Most modern sports have made tremendous strides - through rules changes, equipment enhancements, and education - to minimize the occurrence of catastrophic head injury. Nevertheless, concussions remain a significant problem in all sports. A considerable amount of time and effort has been -
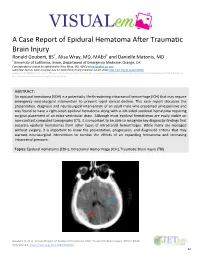
VISUAL Em a Case Report of Epidural Hematoma After Traumatic
VISUAL em A Case Report of Epidural Hematoma After Traumatic Brain Injury * * Ronald Goubert, BS , Alisa Wray, MD, MAEd and Danielle Matonis, MD *University of Goubert R, et al. A Case Report of Epidural Hematoma After Traumatic Brain Injury. JETem 2020. 5(3):V22-24. https://doi.org/10.21980/J8R059 22 VISUAL em Introduction: hematoma (blue arrow), and bilateral subarachnoid Head injury is the leading cause of death and disability in hemorrhages. No skull fractures were noted. children and young adults.1Of the different types of ICH, epidural hematomas (EDH) account for 2%.7-4% of all traumatic Discussion: brain injuries; these are more likely to occur in the second An EDH occurs When there is an injury to a middle meningeal decade of life.2 Although ICHs haVe a mortality of 40% Within artery or vein, diploic vein, or venous sinus. An injury to one of one month of presentation, EDHs are typically associated With these Vessels Will result in blood accumulation between the better outcomes and a mortality of less than 10% if they are inner skull and the dura mater.2,8 Arterial injury, Which occurs in quickly identified and treated.3,4,5,6 These patients are also at an 85% of EDHs, leads to a high pressure bleed that rapidly increased risk of deVeloping long-term neuropsychological expands and compresses the surrounding brain parenchyma.2 sequelae such as headaches, seizures, cognitiVe impairment, Classically, an EDH is associated With a lucid interval after injury and depression.7 Early recognition and proper management of in which patients are asymptomatic for minutes to hours.2,9 an EDH is critical in reducing morbidity and mortality associated During this time, blood collects in the epidural space before with traumatic head injuries. -

Neuro Vignettes Psychiatry Board Exam Preparation Resource
Neuro Vignettes Psychiatry Board Exam Preparation Resource www.BeatTheBoards.com 1 of 69 877-225-8384 Table of Contents 1. Parkinson’s Disease 2. Wilson’s Disease 3. Huntington’s Disease 4. Alzheimer’s Disease 5. Frontal-Temporal Dementia 6. Dementia with Lewy Bodies 7. Binswanger’s Dementia 8. New Variant Creutzfeldt-Jakob Disease 9. Tay Sach’s Disease 10. Friedrich’s Ataxia 11. Metachromatic Leukodystrophy 12. Coma 13. Subdural Hematoma 14. Epidural Hematoma 15. Cortical Ischemic Stroke 16. Brainstem Ischemic Stroke 17. Hemorrhagic Stroke 18. Status Epilepticus 19. Partial Complex Seizure 20. Grand Mal Seizure 21. Multiple Sclerosis 22. Amyotrophic Lateral Sclerosis 23. Guillain-Barre Syndrome 24. Myasthenia Gravis 25. Duchenne Muscular Dystrophy 26. High Grade Glioma 27. Astrocytoma 28. Medulloblastoma 29. Brain Death Evaluation Copyright Notice: Copyright © 2008-2010 American Physician Institute for Advanced Professional Studies, LLC. All rights reserved. This manuscript may not be transmitted, copied, reprinted, in whole or in part, without the express written permission of the copyright holder. Requests for permission or further information should be addressed to Jack Krasuski at: [email protected] or American Physician Institute for Advanced Professional Studies, LLC, 125 Windsor Dr., Suite 111, Oak Brook, IL 60523 Disclaimer Notice: This publication is designed to provide general educational advice. It is provided to the reader with the understanding that Jack Krasuski and American Physician Institute for Advanced Professional Studies LLC are not rendering medical services and are not affiliated with the American Board of Psychiatry and Neurology. If medical or other expert assistance is required, the services of a medical or other consultant should be obtained. -

Northbay Center for Neuroscience “Focus on TBI and Concussion”
NorthBay Center for Neuroscience “Focus on TBI and Concussion” Essential Elements in the Evaluation and Care of the TBI and Concussion Patient May 3, 2019 Edie E. Zusman, MD, MBA Medical Director NorthBay Center for Neuroscience Chief of Neurosurgery Disclosure • Partner – Benzil Zusman, LLC – Neuroscience Strategy Consulting • World Neurosurgery Editorial Board- Section Editor • Neurosurgery – Editorial Board • Board of Directors – Epilepsy Foundation of Northern CA and Head Royce • NorthBay – Foundation Board • International Advisor, RISE Clinic Anambra , Nigeria NorthBay Neurosurgery Team • Corey Beausoleil • Kawanaa Carter • Bita Joobbani • Patrick Maloney • Saint-Aaron Morris • Phillip Parry • Atul Patel • Sherry Taylor • Edie Zusman NorthBay State of the Art Neurosurgery OR Intraoperative CT/O ARM with Computer Guidance Zeiss Pantera Microscope NorthBay Hospital – Top Technology Wellness Center – HealthSpring Fitness o State of the art Operating Rooms o NorthBay is completiing 200 million dollar expansion o Zeiss Microscopes and Endoscopes o Minimally Invasive technology o Stealth Computer Navigation o State of the art Health Fitness Center o 50,000 square foot wellness center o 4 swimming pools and jogging track o Mayo Clinic Model – One Stop Shop o Concussion Patients Seen within Days o Multidisciplinary TBI and Concussion Clinic IRB Approved Clinical Trial for Chronic TBI NorthBay Center for Neuroscience “Focus on TBI and Concussion” Welcome Honored Guests • Diana Lopez Lomeli, MA • Joanne Jacob, RN • Richard Riemer, DO • Mary -

200 Questions Percent 01
SUBSPECIALTY CERTIFICATION EXAMINATION IN VASCULAR NEUROLOGY 2016 Content Blueprint (December 9, 2015) Number of questions: 200 questions Percent 01. Basic science aspects of vascular neurology 4-6% 02. Risk factors and epidemiology 8-12% 03. Clinical features of cerebrovascular diseases 8-12% 04. Evaluation of the patient with cerebrovascular disease 13-17% 05. Causes of stroke 18-22% 06. Complications of stroke 4-6% 07. Treatment of patients with stroke 28-32% 08. Recovery, regenerative approaches, and rehabilitation 4-6% TOTAL 100% Note: A more detailed content outline is shown below 2016 ABPN Content Specifications Page 1 of 16 Posted: December 21, 2015 Subspecialty Certification in Vascular Neurology SUBSPECIALTY CERTIFICATION EXAMINATION IN VASCULAR NEUROLOGY 2016 Content Outline Content Areas 01. Basic science aspects of vascular neurology A. Vascular neuroanatomy 1. Extracranial arterial anatomy 2. Intracranial arterial anatomy 3. Collaterals 4. Alterations of vascular anatomy 5. Venous anatomy 6. Spinal cord vascular anatomy 7. Specific vascular-brain anatomic correlations 8. End vessel syndromes B. Stroke pathophysiology 1. Cerebral blood flow a. Vascular smooth muscle control b. Vasodilation and vasoconstriction c. Autoregulation d. Vasospasm e. Rheology f. Blood flow in stroke 2. Blood-brain barrier in stroke 3. Coagulation cascade a. Clotting factors b. Platelet function c. Endothelium function d. Biochemical factors 4. Metabolic and cellular consequences of ischemia a. Ischemic cascade b. Reperfusion changes c. Electrophysiology d. Gene regulation 5. Inflammation and stroke 6. Brain edema and increased ICP 2016 ABPN Content Specifications Page 2 of 16 Posted: December 21, 2015 Subspecialty Certification in Vascular Neurology a. Secondary effects 7. Restoration and recovery following stroke 8.