Isolation of Functionally Distinct Mesenchymal Stem Cell Subsets Using Antibodies Against CD56, CD271, and Mesenchymal Stem Cell Antigen-1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Flow Reagents Single Color Antibodies CD Chart
CD CHART CD N° Alternative Name CD N° Alternative Name CD N° Alternative Name Beckman Coulter Clone Beckman Coulter Clone Beckman Coulter Clone T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells CD1a T6, R4, HTA1 Act p n n p n n S l CD99 MIC2 gene product, E2 p p p CD223 LAG-3 (Lymphocyte activation gene 3) Act n Act p n CD1b R1 Act p n n p n n S CD99R restricted CD99 p p CD224 GGT (γ-glutamyl transferase) p p p p p p CD1c R7, M241 Act S n n p n n S l CD100 SEMA4D (semaphorin 4D) p Low p p p n n CD225 Leu13, interferon induced transmembrane protein 1 (IFITM1). p p p p p CD1d R3 Act S n n Low n n S Intest CD101 V7, P126 Act n p n p n n p CD226 DNAM-1, PTA-1 Act n Act Act Act n p n CD1e R2 n n n n S CD102 ICAM-2 (intercellular adhesion molecule-2) p p n p Folli p CD227 MUC1, mucin 1, episialin, PUM, PEM, EMA, DF3, H23 Act p CD2 T11; Tp50; sheep red blood cell (SRBC) receptor; LFA-2 p S n p n n l CD103 HML-1 (human mucosal lymphocytes antigen 1), integrin aE chain S n n n n n n n l CD228 Melanotransferrin (MT), p97 p p CD3 T3, CD3 complex p n n n n n n n n n l CD104 integrin b4 chain; TSP-1180 n n n n n n n p p CD229 Ly9, T-lymphocyte surface antigen p p n p n -

Multiomics of Azacitidine-Treated AML Cells Reveals Variable And
Multiomics of azacitidine-treated AML cells reveals variable and convergent targets that remodel the cell-surface proteome Kevin K. Leunga, Aaron Nguyenb, Tao Shic, Lin Tangc, Xiaochun Nid, Laure Escoubetc, Kyle J. MacBethb, Jorge DiMartinob, and James A. Wellsa,1 aDepartment of Pharmaceutical Chemistry, University of California, San Francisco, CA 94143; bEpigenetics Thematic Center of Excellence, Celgene Corporation, San Francisco, CA 94158; cDepartment of Informatics and Predictive Sciences, Celgene Corporation, San Diego, CA 92121; and dDepartment of Informatics and Predictive Sciences, Celgene Corporation, Cambridge, MA 02140 Contributed by James A. Wells, November 19, 2018 (sent for review August 23, 2018; reviewed by Rebekah Gundry, Neil L. Kelleher, and Bernd Wollscheid) Myelodysplastic syndromes (MDS) and acute myeloid leukemia of DNA methyltransferases, leading to loss of methylation in (AML) are diseases of abnormal hematopoietic differentiation newly synthesized DNA (10, 11). It was recently shown that AZA with aberrant epigenetic alterations. Azacitidine (AZA) is a DNA treatment of cervical (12, 13) and colorectal (14) cancer cells methyltransferase inhibitor widely used to treat MDS and AML, can induce interferon responses through reactivation of endoge- yet the impact of AZA on the cell-surface proteome has not been nous retroviruses. This phenomenon, termed viral mimicry, is defined. To identify potential therapeutic targets for use in com- thought to induce antitumor effects by activating and engaging bination with AZA in AML patients, we investigated the effects the immune system. of AZA treatment on four AML cell lines representing different Although AZA treatment has demonstrated clinical benefit in stages of differentiation. The effect of AZA treatment on these AML patients, additional therapeutic options are needed (8, 9). -

PSGL-1 (KPL1): Sc-13535
SAN TA C RUZ BI OTEC HNOL OG Y, INC . PSGL-1 (KPL1): sc-13535 BACKGROUND STORAGE PSGL-1 (P-Selectin glycoprotein ligand, also designated CD162), exists as Store at 4° C, **DO NOT FREEZE**. Stable for one year from the date of a disulfide-linked homodimer. PSGL-1 is a type 1 membrane protein that shipment. Non-hazardous. No MSDS required. localizes on the tips of microvilli of leukocytes. Its extracellular domain is rich in serines, threonines and prolines, and includes a series of 15 and 16 DATA ecameric repeats in HL-60 and U-937 cells, and human leukocytes, respec - A B tively. Although PSGL-1 appears to be the sole receptor for P-Selectin on ABCD human hematopoietic cells, it also interacts with E-Selectin through a unique binding site. In order to bind PSGL-1 to either E-Selectin or P-Selectin, PSGL-1 215K– must be sialylated and fucosylated. PSLG-1 is a mucin-like molecule, much PSGL-1 like leukosialin (CD43), CD164 and CD34. These proteins belong to an emerg - ing family of cell adhesion receptors called sialomucins, which transduce negative signals in hematopoietic cells. 114K– CHROMOSOMAL LOCATION PSGL-1 (KPL1) HRP: sc-13535 HRP. Direct western PSGL-1 (KPL1): sc-13535. Immunofluorescence staining blot analysis of PSGL-1 expression in MOLT-4 (A), of methanol-fixed CCRF-CEM cells showing membrane Genetic locus: SELPLG (human) mapping to 12q24.11. AML-193 (B), Jurkat (C) and CCRF-CEM (D) whole staining ( A). Immunoperoxidase staining of formalin cell lysates. fixed, paraffin-embedded human lymph node tissue showing membrane and cytoplasmic staining of cells SOURCE in non-germinal center ( B). -

Genome-Wide Gene Expression Profiles of Ovarian Carcinoma: Identification of Molecular Targets for the Treatment of Ovarian Carcinoma
365-384 30/3/2009 09:55 Ì Page 365 MOLECULAR MEDICINE REPORTS 2: 365-384, 2009 365 Genome-wide gene expression profiles of ovarian carcinoma: Identification of molecular targets for the treatment of ovarian carcinoma DRAGOMIRA NIKOLAEVA NIKOLOVA1,4, NIKOLAI DOGANOV2, RUMEN DIMITROV2, KRASIMIR ANGELOV3, SIEW-KEE LOW1, IVANKA DIMOVA4, DRAGA TONCHEVA4, YUSUKE NAKAMURA1 and HITOSHI ZEMBUTSU1 1Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo, Tokyo 108-8639, Japan; 2University Hospital of Obstetrics and Gynecology ‘Maichin Dom’, Sofia 1431; 3National Hospital of Oncology, Sofia 1233; 4Department of Medical Genetics, Medical University of Sofia, Sofia 1431, Bulgaria Received October 31, 2008; Accepted January 7, 2009 DOI: 10.3892/mmr_00000109 Abstract. This study aimed to clarify the molecular mecha- in women. As there are no specific indicators or symptoms of nisms involved in ovarian carcinogenesis, and to identify ovarian cancer during the early stages of the disease, the candidate molecular targets for its diagnosis and treatment. majority of patients with epithelial ovarian cancer (EOC) are The genome-wide gene expression profiles of 22 epithelial diagnosed at an advanced stage, with involvement of other ovarian carcinomas were analyzed with a microarray represent- sites such as the upper abdomen, pleural space and paraaortic ing 38,500 genes, in combination with laser microbeam lymph nodes. The cancer antigen 125 assay (CA-125) has been microdissection. A total of 273 commonly up-regulated used to screen for ovarian cancer, but is not specific; only 50% transcripts and 387 down-regulated transcripts were identified of patients with early ovarian cancer test positive using this in the ovarian carcinoma samples. -
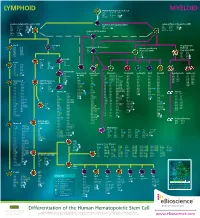
Lymphoid Myeloid
LYMPHOID Human Hematopoietic Stem Cell MYELOID CD34 CD117 (c-kit) CD338 CD38low/neg CD133 linneg CD59 CD135 (Flt3) GATA2 CD90 (Thy1) CD164 TdT Common Lymphoid Progenitor (CLP) Multi-Potent Progenitor (MPP) Common Myeloid Progenitor (CMP) low neg CD33 CD123low CD174 CD7 CD117 (c-kit) C/EBPα CD34 linneg neg CD34 CD131 Ikaros CD10 CD127 GATA2 CD135 (Flt3) TdT CD24neg CD135 (Flt3) GATA3 CD45RA CD164 PU.1 CD34 CD164 PU.1high Common DC Progenitor CD117 (c-kit) CD173 CD38 HLA-DR TdT CD45RA Aiolos CD11c dim CD90low c-mybhigh CD33 Pro-B Pre-NK/T Megakaryocyte- CD10 CD124 CD5 Conventional DC Precursor Erythroid CD19 CD164 CD34 CD11c Granulocyte-Myeloid CD20 CD252 CD14neg Progenitor (GMP) Progenitor (MEP) CD22 CD268 CD33dim CD33 CD131 CD34neg CD275 CD34 CD45RA CD34 CD164 CD72 HLA-DR CD36 FOG-1 CD74 CD123 PU.1 CD110 GATA1 CD123high GATA2 Pre-T Pro-NK CD7 Pre-B CD1a CD28 CD10neg CD2 CD34 neg CD10 CD124 CD34 CD3 CD45 CD117 (c-kit) CD19 CD164 CD5 CD127 (IL-7Rα) CD20 CD252 CD7high NOTCH1 CD22 CD268 CD34neg CD275 CD72 HLA-DR Immature NK CD74 IgM CD34neg Plasmacytoid Conventional Monocyte Neutrophil Eosinophil Mast Basophil Megakaryocyte Erythrocyte CD79a Pax5 CD94neg DC (pDC) DC (cDC) CD4low CD85a (ILT5) CD171 CD10 CD9 CD9 CD9 CD41 CD51 CD35 CD236 CD117 (c-kit) CD9 CD85d (ILT4) CD172a (SIRPα) CD15 CD11b CD11b CD11a CD42a CD61 CD44 CD236R CD4 CD1c CD122 CD11b CD85h (ILT1) CD172b CD16b CD15 CD15 CD11b CD42b CD110 CD123 CD238 CD11c neg CD1d CD161 CD11c CD85j (ILT2) CD180 CD17 CD24 CD24 CD13 CD42c CD112 CD173 CD239 CD45RA CD2 Ets-1 CD13 -

Mouse CD Marker Chart Bdbiosciences.Com/Cdmarkers
BD Mouse CD Marker Chart bdbiosciences.com/cdmarkers 23-12400-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1d CD1.1, CD1.2, Ly-38 Lipid, Glycolipid Ag + + + + + + + + CD104 Integrin b4 Laminin, Plectin + DNAX accessory molecule 1 (DNAM-1), Platelet and T cell CD226 activation antigen 1 (PTA-1), T lineage-specific activation antigen 1 CD112, CD155, LFA-1 + + + + + – + – – CD2 LFA-2, Ly-37, Ly37 CD48, CD58, CD59, CD15 + + + + + CD105 Endoglin TGF-b + + antigen (TLiSA1) Mucin 1 (MUC1, MUC-1), DF3 antigen, H23 antigen, PUM, PEM, CD227 CD54, CD169, Selectins; Grb2, β-Catenin, GSK-3β CD3g CD3g, CD3 g chain, T3g TCR complex + CD106 VCAM-1 VLA-4 + + EMA, Tumor-associated mucin, Episialin + + + + + + Melanotransferrin (MT, MTF1), p97 Melanoma antigen CD3d CD3d, CD3 d chain, T3d TCR complex + CD107a LAMP-1 Collagen, Laminin, Fibronectin + + + CD228 Iron, Plasminogen, pro-UPA (p97, MAP97), Mfi2, gp95 + + CD3e CD3e, CD3 e chain, CD3, T3e TCR complex + + CD107b LAMP-2, LGP-96, LAMP-B + + Lymphocyte antigen 9 (Ly9), -

Supplementary Materials
Supplementary materials Supplementary Text Figs. S1 to S19, Table S1 to S8, Data set 1 Fig. S1. Differential expression of known 69 tumor initiating genes in human normal brain and GBM tissue samples. (A) RNA sequencing analysis. (Upper) Venn diagram representing the number of transcripts (FPKM > 1) up- or down-regulated in human glioblastoma patient samples. (lower) Volcano plots displaying differentially 1 expressed genes. G/N, mean values derived from 20 human glioblastoma,G, samples divided by values from 19 human normal, N, brain tissues. In plot, red dots denote genes that undergo a statistically significant 10-fold change in G/N, either up or down (P < 0.01). Red-labeled dots have G/N ratios greater than 10-fold higher. Blue dot- labeled genes have G/N ratios between 2 to 10. (B) Data are shown as mean ± standard deviation for at least 19 independent specimens. Data are mean ± SD. P value by unpaired 2-tailed Student’s t test 2 Fig. S2. High grade glioma exhibits high CD133 and CD44 markers expression. (A) Low- and high- grade glioma tissue images stained with indicated antibodies were supported by the Human protein atlas (http://www.proteinatlas.org/) or obtained by staining of tissue microarray paraffin sections with CXCR4 antibody (last Panel). (B) Images in fig. S2A were quantified by Image J software. Data are mean ± SD (N>3). P value by unpaired 2-tailed Student’s t test. (C) Tissue microarray paraffin sections from human glioblastoma tissues and normal brain tissues were subjected to immunofluorescent staining using antibodies against CXCR4, CD44, or CD133. -

Adenosine Signature Genes Associate with Tumor Regression in Renal Cell Carcinoma
PRAME TNFSF18 CXCL1 CXCL6 BST1 CLEC5A PSMD7 DMBT1 40 CEACAM6 FOXJ1 CXCR1 CXCR2 ALCAM 20 ITCH Adenosine signature genes associate with tumor regression in renal cell carcinoma CXCL3 CXCL5 C2 CSF1 STAT2 0 MAP2K2 (RCC) patients treated with the adenosine A2A receptor (A2AR) antagonist, CPIADA -444. IRAK4 CT45A1 1 1 2 3 4 5 6 7 CCR6 7 8 9 Andrew Hotson, Stephen Willingham, −20 Lawrence Fong, John Powderly II, Jason Luke, Mario Sznol, Saby George, Toni K. Choueiri, C9 Marios Giannakis, Brian Rini, Shivaani Kummar, 10 1 1 1 1 1 1 LBP Erik Evensen, Ian McCaffery, Chunyan Gu, Long Kwei, Ginna Laport, Joe Buggy and Richard Miller TLR5 TNFSF14 CFD 1 −40 2 3 STAT3 4 5 Corvus Pharmaceuticals, Burlingame, CA; University of California, San Francisco, San Francisco, CA; Carolina BioOncology Institute, Huntersville, NC; BCL6 University of Chicago Medical Center, Chicago, IL, Yale 6 7 8 CXCR4 9 University School of Medicine, New Haven, CT; Roswell Park, Buffalo, NY; Dana-Farber Cancer Institute, Boston, MA; Cleveland Clinic, Cleveland, OH;CCL20 Stanford University School of Medicine, Stanford, CA; CEBPB 10 LIF Basis Bioscience, Foster City, CA−60 SAA1 C1R C1S PTGS2 PRAME C4BPA TNFSF18 Background CXCL1 CCL11 Adenosine Signature and Co-expressed Genes Identified in Patient Subset by Unsupervised ClusteringCXCL6 CXCL2 BST1 adeno_sig CLEC5A 40 A separate patient subset is low for adenosine signature and expresses alternate biological pathwaysTREM1 PSMD7 • DMBT1 Adenosine blocks T-cell activation and promotes myeloid SLC11A1 CEACAM6 TNFRSF11A FOXJ1 20 suppression -

CD System of Surface Molecules
THE CD SYSTEM OF LEUKOCYTE APPENDIX 4A SURFACE MOLECULES Monoclonal Antibodies to Human Cell Surface Antigens APPENDIX 4A Alice Beare,1 Hannes Stockinger,2 Heddy Zola,1 and Ian Nicholson1 1Women’s and Children’s Health Research Institute, Women’s and Children’s Hospital, Adelaide, Australia 2Institute of Immunology, University of Vienna, Vienna ABSTRACT Many of the leukocyte cell surface molecules are known by “CD” numbers. In this Appendix, a short introduction describes the history and the use of CD nomenclature and provides a few key references to enable access to the wider literature. This is followed by a table that lists all human molecules with approved CD names, tabulating alternative names, key structural features, cellular expression, major known functions, and usefulness of the molecules or antibodies against them in research or clinical applications. Curr. Protoc. Immunol. 80:A.4A.1-A.4A.73. C 2008 by John Wiley & Sons, Inc. Keywords: CD nomenclature r HLDA r HCDM r leukocyte marker r human leukocyte differentiation r antigens INTRODUCTION During the last 25 years, large numbers of monoclonal antibodies (MAbs) have been pro- duced that have facilitated the purification and functional characterization of a plethora of leukocyte surface molecules. The antibodies have been even more useful as markers for cell populations, allowing the counting, separation, and functional study of numer- ous subsets of cells of the immune system. A series of international workshops were instrumental in coordinating this development through multi-laboratory “blind” studies of thousands of antibodies. These HLDA (Human Leukocyte Differentiation Antigens) Workshops have, up until now, defined 500 different entities and assigned them cluster of differentiation (CD) designations. -

Domain of the CD164 Receptor Proliferation Interact with the First
CD164 Monoclonal Antibodies That Block Hemopoietic Progenitor Cell Adhesion and Proliferation Interact with the First Mucin Domain of the CD164 Receptor This information is current as of September 28, 2021. Regis Doyonnas, James Yi-Hsin Chan, Lisa H. Butler, Irene Rappold, Jane E. Lee-Prudhoe, Andrew C. W. Zannettino, Paul J. Simmons, Hans-Jörg Bühring, Jean-Pierre Levesque and Suzanne M. Watt J Immunol 2000; 165:840-851; ; Downloaded from doi: 10.4049/jimmunol.165.2.840 http://www.jimmunol.org/content/165/2/840 http://www.jimmunol.org/ References This article cites 41 articles, 27 of which you can access for free at: http://www.jimmunol.org/content/165/2/840.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. CD164 Monoclonal Antibodies That Block Hemopoietic Progenitor Cell Adhesion and Proliferation Interact with the First Mucin Domain of the CD164 Receptor1 Regis Doyonnas,* James Yi-Hsin Chan,* Lisa H.