Aloysiae Citriodorae Folium
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Antibacterial and Antioxidant Effects of Clove (Syzygium Aromaticum) and Lemon Verbena (Aloysia Citriodora) Essential Oils
Journal of Human, Environment, and Health Promotion. 2019; 5(2): 86-93 Journal of Human, Environment, and Health Promotion Journal homepage: www.zums.ac.ir/jhehp The Antibacterial and Antioxidant Effects of Clove (Syzygium aromaticum) and Lemon Verbena (Aloysia citriodora) Essential Oils a b * c d Mahzad Hosseini Abdollah Jamshidi Mojtaba Raeisi Mohammad Azizzadeh a Student of Food Hygiene, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. b Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. c Department of Nutrition, Faculty of Health, Golestan University of Medical sciences, Gorgan, Iran. d Department of Clinical Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. *Corresponding author: Abdollah Jamshidi Department of Food Hygiene and Aquaculture, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. Postal code: 9187195786. E-mail address: [email protected] ARTICLE INFO ABSTARACT Article type: Background: The study aimed to investigate the chemical composition, antimicrobial Original article effects, and antioxidant properties of clove and lemon verbena essential oils (EOs). Article history: Methods: The chemical composition of the EOs was identified using gas Received 31 March 2019 chromatography/mass spectrometry (GC/MS). In addition, the antibacterial effects of EOs Revised 9 June 2019 against seven important foodborne bacteria were assessed using the disk-diffusion, agar Accepted 20 June 2019 well-diffusion, and broth microdilution assays. Evaluation of the antioxidant properties of the EOs was carried out using DPPH, β-carotene-linoleic acid bleaching, and reducing DOI: 10.29252/jhehp.5.2.7 power assay. Keywords: Results: All the tested bacteria demonstrated susceptibility to EOs, with the highest Essential oil susceptibility observed in Bacillus cereus to the clove EO in the agar disk-diffusion test. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -
Show Activity
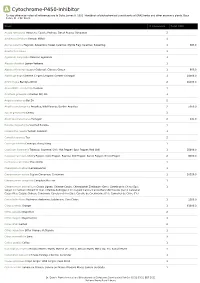
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Redalyc.Meiotic Behavior and Pollen Viability of Aloysia Gratissima and Aloysia Triphylla (Verbenaceae)
Ciência e Natura ISSN: 0100-8307 [email protected] Universidade Federal de Santa Maria Brasil Lenz Hister, Carmine Aparecida; Bosio Tedesco, Solange; Ferreira da Silva, Antonio Carlos; Scotti do Canto-Dorow, Thais Meiotic behavior and pollen viability of Aloysia gratissima and Aloysia triphylla (Verbenaceae) Ciência e Natura, vol. 32, núm. 1, 2010, pp. 37-47 Universidade Federal de Santa Maria Santa Maria, Brasil Available in: http://www.redalyc.org/articulo.oa?id=467546357003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Meiotic behavior and pollen viability of Aloysia gratissima and Aloysia triphylla (Verbenaceae) Carmine Aparecida Lenz Hister, Solange Bosio Tedesco, Antonio Carlos Ferreira da Silva, Thais Scotti do Canto-Dorow Departamento de Biologia/CCNE Universidade Federal de Santa Maria/Santa Maria, RS e-mail: [email protected] Abstract The use of medicinal plants for the treatment of diseases that attack human beings has been a practice for centuries and it is seen as one of the main therapeutic resource in many communities and ethnical groups, especially in developing countries. In Brazil, the economic potential of the germoplasm of medicinal plants is a wealth to be used and preserved. Native and exotic species are used medicinally in Brazil. Among them, Aloysia gratissima (Gill et Hook) Tronc. (native) and Aloysia triphylla (LHer.) Britton (exotic) both from Verbenaceae Family are highlighted. In this study the meiotic behavior and pollen viability in populations of these species of the genus Aloysia from Rio Grande do Sul State were analyzed. -

Industrial Crops and Products 77 (2015) 972–982
Industrial Crops and Products 77 (2015) 972–982 Contents lists available at ScienceDirect Industrial Crops and Products journal homepage: www.elsevier.com/locate/indcrop Extending the use of irradiation to preserve chemical and bioactive properties of medicinal and aromatic plants: A case study with four species submitted to electron beam a a b a Eliana Pereira , Amilcar L. Antonio , Andrzej Rafalski , João C.M. Barreira , a a,∗ Lillian Barros , Isabel C.F.R. Ferreira a Centro de Investigac¸ ão de Montanha (CIMO), ESA, Instituto Politécnico de Braganc¸ a, Campus de Santa Apolónia, 1172, 5301-855 Braganc¸ a, Portugal b Centre for Radiation Research and Technology, Institute of Nuclear Chemistry and Technology, Dorodna Str. 16, 03-195 Warsaw, Poland a r t i c l e i n f o a b s t r a c t Article history: The effects of gamma irradiation on Aloysia citrodora, Melissa officinalis, Melittis melissophyllum and Mentha Received 28 June 2015 piperita were previously evaluated. Herein, the same species were treated with electron-beam irradia- Received in revised form tion (EB) and the same parameters were evaluated. Instead of presenting absolute values for each studied 24 September 2015 parameter, data were evaluated as percentage of induced variation. Besides the newly obtained results, Accepted 29 September 2015 data from a previous work was recalled and normalized in the same manner. Several examples of per- centage variations specific to a plant species or irradiation condition were found. Nevertheless, it was not Keywords: possible to identify unequivocal trends. Even so, when evaluated in an integrative way, the parameters Irradiation with highest discriminating ability among irradiation conditions or plant species were fatty acids and Aromatic plants bioactive indicators. -

(Aloysia Citriodora Palau) from Argentina Boletín Latinoamericano Y Del Caribe De Plantas Medicinales Y Aromáticas, Vol
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas ISSN: 0717-7917 [email protected] Universidad de Santiago de Chile Chile Gattuso, Susana; van Baren, Catalina M.; Gil, Alejandra; Bandoni, Arnaldo; Ferraro, Graciela; Gattuso, Martha Morpho-histological and quantitative parameters in the characterization of lemon verbena (Aloysia citriodora palau) from Argentina Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 7, núm. 4, julio, 2008, pp. 190-198 Universidad de Santiago de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=85670402 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © 2008 Los Autores Derechos de Publicación © 2008 Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 7 (4), 190 - 198 BLACPMA ISSN 0717 7917 Artículo Original | Original Article Morpho-histological and quantitative parameters in the characterization of lemon verbena (Aloysia citriodora palau) from Argentina. [Parámetros morfo-histológicos cuantitativos en la caracterización de lemon verbena (Aloysia citriodora palau) de Argentina] Susana GATTUSO1*, Catalina M. van BAREN2, Alejandra GIL3, Arnaldo BANDONI2; Graciela FERRARO2 y Martha GATTUSO1 1. Cátedra de Botánica, Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario Suipacha 531, S2002LRK, Rosario, Argentina. 2. Cátedra de Farmacognosia-IQUIMEFA. Facultad de Farmacia y Bioquímica.UBA-CONICET. Junín 956, 2º piso. C1113AAD Buenos Aires Argentina. 3. Facultad de Agronomía, Universidad de Buenos Aires, Av. San Martín 4453, (C 1417 DSE) Buenos Aires - Argentina. -

A Review on the Chemistry of Some Species of Genus Lippia (Verbenaceae Family)
Journal of Scientific and Innovative Research 2014; 3(4): 460-466 Available online at: www.jsirjournal.com Review Article A review on the chemistry of some species of genus ISSN 2320-4818 Lippia (Verbenaceae family) JSIR 2014; 3(4): 460-466 © 2014, All rights reserved Received: 26-06-2014 Japheth Omollo Ombito*, Elsie Nyangweso Salano, Philemon Kipkirui Yegon, Wesley Accepted: 24-08-2014 Kipkirui Ngetich, Elizabeth Muthoni Mwangi Abstract Japheth Omollo Ombito Department of Chemistry, Egerton Recently, focus on plant research has increased globally and a large amount of evidence has University, P.O. Box 536, Egerton- 20115, Kenya collected to show great potential of medicinal plants employed in diverse traditional systems. In the customary forms of medicine, plants provided a large number of remedies, which were often Elsie Nyangweso Salano useful. Lippia genus, which belongs to the family Verbenaceae yields appreciable quantities of Department of Biochemistry, Egerton University, P.O. Box 536, metabolites some of which have been shown to have valuable biological activities. Many Egerton-20115, Kenya phytochemical investigations done on this genus have shown the presence of various compounds like triterpenoids, phenols, flavonoids, phenylpropanoids and steroids. This review Philemon Kipkirui Yegon Department of Chemistry, Egerton focuses on ethnopharmacology, phytochemistry and pharmacology of Lippia genus to allow the University, P.O. Box 536, Egerton- evaluation of the potential for utilization of the largest biomass of Lippia genus available. 20115, Kenya Wesley Kipkirui Ngetich Keywords: Lippia, Triterpenoids, Phenols, Flavonoids, Phytochemistry, Department of Chemistry, Egerton Pharmacology. University, P.O. Box 536, Egerton- 20115, Kenya Elizabeth Muthoni Mwangi Introduction Department of Chemistry, Egerton University, P.O. -

WRA Species Report
Family: Verbenaceae Taxon: Aloysia citriodora Synonym: Aloysia triphylla (L'Hér.) Britton Common Name: lemon verbena Lippia citrodora Kunth Zitronenstrauch Lippia triphylla (L'Hér.) Kuntze cidrão Verbena triphylla L'Hér. cedrón Zappania citrodora Lam. verveine citronelle Questionaire : current 20090513 Assessor: Chuck Chimera Designation: L Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 0 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 n 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n -

The New York Botanical Garden
Vol. XV DECEMBER, 1914 No. 180 JOURNAL The New York Botanical Garden EDITOR ARLOW BURDETTE STOUT Director of the Laboratories CONTENTS PAGE Index to Volumes I-XV »33 PUBLISHED FOR THE GARDEN AT 41 NORTH QUBKN STRHBT, LANCASTER, PA. THI NEW ERA PRINTING COMPANY OFFICERS 1914 PRESIDENT—W. GILMAN THOMPSON „ „ _ i ANDREW CARNEGIE VICE PRESIDENTS J FRANCIS LYNDE STETSON TREASURER—JAMES A. SCRYMSER SECRETARY—N. L. BRITTON BOARD OF- MANAGERS 1. ELECTED MANAGERS Term expires January, 1915 N. L. BRITTON W. J. MATHESON ANDREW CARNEGIE W GILMAN THOMPSON LEWIS RUTHERFORD MORRIS Term expire January. 1916 THOMAS H. HUBBARD FRANCIS LYNDE STETSON GEORGE W. PERKINS MVLES TIERNEY LOUIS C. TIFFANY Term expire* January, 1917 EDWARD D. ADAMS JAMES A. SCRYMSER ROBERT W. DE FOREST HENRY W. DE FOREST J. P. MORGAN DANIEL GUGGENHEIM 2. EX-OFFICIO MANAGERS THE MAYOR OP THE CITY OF NEW YORK HON. JOHN PURROY MITCHEL THE PRESIDENT OP THE DEPARTMENT OP PUBLIC PARES HON. GEORGE CABOT WARD 3. SCIENTIFIC DIRECTORS PROF. H. H. RUSBY. Chairman EUGENE P. BICKNELL PROF. WILLIAM J. GIES DR. NICHOLAS MURRAY BUTLER PROF. R. A. HARPER THOMAS W. CHURCHILL PROF. JAMES F. KEMP PROF. FREDERIC S. LEE GARDEN STAFF DR. N. L. BRITTON, Director-in-Chief (Development, Administration) DR. W. A. MURRILL, Assistant Director (Administration) DR. JOHN K. SMALL, Head Curator of the Museums (Flowering Plants) DR. P. A. RYDBERG, Curator (Flowering Plants) DR. MARSHALL A. HOWE, Curator (Flowerless Plants) DR. FRED J. SEAVER, Curator (Flowerless Plants) ROBERT S. WILLIAMS, Administrative Assistant PERCY WILSON, Associate Curator DR. FRANCIS W. PENNELL, Associate Curator GEORGE V. -

Mexican Oregano Lippia Graveolens
ARIZONA-SONORA DESERT MUSEUM PLANT CARE INFORMATION Mexican Oregano Lippia graveolens DESCRIPTION: Mexican Oregano is native to Texas and southern New Mexico, Mexico and Central America as far south as Nicaragua. It is a twiggy shrub, reaching 3 to 6 feet tall and as wide. Dark green leaves are high in oils, giving the plant a flavor similar to oregano. The leaves are widely used as an herb in Mexico and Central America. The flavor has notes of lemon and camphor, which adds a complexity to sauces. It also makes great addition to seafood, cheeses and vegetables. Fragrant buttery-colored flowers with yellow centers can be found on the plant throughout the year, especially after rains. Another attraction of this plant is that the flowers attract many species of butterflies. RECOMMENDED USE: Rock garden, enhanced desert revegetation. Plant it where the aroma of the foliage can be enjoyed. Mexican Oregano is a flavor filled culinary herb that is used extensively in Mexican and Tex-Mex cooking. It can be grown as an annual or containerized for indoor winter growing. CULTURE: Hardiness: It is frost hardy to around 20oF with little damage. Sun tolerance: This plant thrives in full sun or lightly filtered sun. Too much shade will make it bloom less and grow leggy. Watering and feeding: This versatile herb grows in dry climates and uses minimal water once established. Watch watering closely during the first hot season. After establishment, a weekly watering will help it look its best. Feeding seldom needed unless grown in a container. Soil requirements: Any soil. -

Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals As Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity
pathogens Article Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity Chrysa Androutsopoulou 1, Spyridoula D. Christopoulou 2, Panagiotis Hahalis 3, Chrysoula Kotsalou 1, Fotini N. Lamari 2 and Apostolos Vantarakis 1,* 1 Department of Public Health, Faculty of Medicine, University of Patras, 26504 Patras, Greece; [email protected] (C.A.); [email protected] (C.K.) 2 Laboratory of Pharmacognosy & Chemistry of Natural Products, Department of Pharmacy, University of Patras, 26504 Patras, Greece; [email protected] (S.D.C.); [email protected] (F.N.L.) 3 Tentoura Castro-G.P. Hahalis Distillery, 26225 Patras, Greece; [email protected] * Correspondence: [email protected] Abstract: Essential oils (EOs) and extracts of rose geranium (Pelargonium graveolens) and petals of rose (Rosa damascena) have been fully characterized in terms of composition, safety, antimicrobial, and antiviral properties. They were analyzed against Escherichia coli, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, Aspergillus niger, and Adenovirus 35. Their toxicity and life span were also determined. EO of P. graveolens (5%) did not retain any antibacterial activity (whereas at Citation: Androutsopoulou, C.; 100% it was greatly effective against E. coli), had antifungal activity against A. niger, and significant Christopoulou, S.D.; Hahalis, P.; antiviral activity. Rose geranium extract (dilutions 25−90%) (v/v) had antifungal and antibacterial Kotsalou, C.; Lamari, F.N.; Vantarakis, activity, especially against E. coli, and dose-dependent antiviral activity. Rose petals EO (5%) retains A. Evaluation of Essential Oils and low inhibitory activity against S. aureus and S. Typhimurium growth (about 20−30%), antifungal Extracts of Rose Geranium and Rose activity, and antiviral activity for medium to low virus concentrations. -

Oregano: the Genera Origanum and Lippia
Part 5 Pharmacology © 2002 Taylor & Francis 8 The biological/pharmacological activity of the Origanum Genus Dea Bariˇceviˇc and Tomaˇz Bartol INTRODUCTION In the past, several classifications were made within the morphologically and chemically diverse Origanum (Lamiaceae family) genus. According to different taxonomists, this genus comprises a different number of sections, a wide range of species and subspecies or botanical varieties (Melegari et al., 1995; Kokkini, 1997). Respecting Ietswaart taxo- nomic revision (Tucker, 1986; Bernath, 1997) there exist as a whole 49 Origanum taxa within ten sections (Amaracus Bentham, Anatolicon Bentham, Brevifilamentum Ietswaart, Longitubus Ietswaart, Chilocalyx Ietswaart, Majorana Bentham, Campanulaticalyx Ietswaart, Elongatispica Ietswaart, Origanum Ietswaart, Prolaticorolla Ietswaart) the major- ity of which are distributed over the Mediterranean. Also, 17 hybrids between different species have been described, some of which are known only from artificial crosses (Kokkini, 1997). Very complex in their taxonomy, Origanum biotypes vary in respect of either the content of essential oil in the aerial parts of the plant or essential oil compos- ition. Essential oil ‘rich’ taxa with an essential oil content of more than 2 per cent (most commercially known oregano plants), is mainly characterised either by the domi- nant occurrence of carvacrol and/or thymol (together with considerable amounts of ␥-terpinene and p-cymene) or by linalool, terpinene-4-ol and sabinene hydrate as main components (Akgül and Bayrak, 1987; Tümen and Bas¸er, 1993; Kokkini, 1997). The Origanum species, which are rich in essential oils, have been used for thousands of years as spices and as local medicines in traditional medicine. The name hyssop (the Greek form of the Hebrew word ‘ezov’), that is called ‘za’atar’ in Arabic and origanum in Latin, was first mentioned in the Bible (Exodus 12: 22 description of the Passover ritual) (Fleisher and Fleisher, 1988).