Molecules 2001, 6, 736-769 Molecules ISSN 1420-3049 Review
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

The Great Tekhelet Debate—Blue Or Purple? Baruch and Judy Taubes Sterman
archaeological VIEWS The Great Tekhelet Debate—Blue or Purple? Baruch and Judy Taubes Sterman FOR ANCIENT ISRAELITES, TEKHELET WAS writings of rabbinic scholars and Greek and Roman God’s chosen color. It was the color of the sumptu- naturalists had convinced Herzog that tekhelet was a ous drapes adorning Solomon’s Temple (2 Chroni- bright sky-blue obtained from the natural secretions cles 3:14) as well as the robes worn by Israel’s high of a certain sea snail, the Murex trunculus, known to priests (Exodus 28:31). Even ordinary Israelites produce a dark purple dye.* were commanded to tie one string of tekhelet to But the esteemed chemist challenged Herzog’s the corner fringes (Hebrew, tzitzit) of their gar- contention: “I consider it impossible to produce a ments as a constant reminder of their special rela- pure blue from the purple snails that are known to Tekhelet was tionship with God (Numbers 15:38–39). me,” Friedländer said emphatically.1 But how do we know what color the Biblical writ- Unfortunately, neither Herzog nor Friedländer God’s chosen ers had in mind? While tekhelet-colored fabrics and lived to see a 1985 experiment by Otto Elsner, a color. It colored clothes were widely worn and traded throughout the chemist with the Shenkar College of Fibers in Israel, ancient Mediterranean world, by the Roman period, proving that sky-blue could, in fact, be produced the drapes donning tekhelet and similar colors was the exclusive from murex dye. During a specific stage in the dyeing of Solomon’s privilege of the emperor. -

Megillat Esther
The Steinsaltz Megillot Megillot Translation and Commentary Megillat Esther Commentary by Rabbi Adin Even-Israel Steinsaltz Koren Publishers Jerusalem Editor in Chief Rabbi Jason Rappoport Copy Editors Caryn Meltz, Manager The Steinsaltz Megillot Aliza Israel, Consultant Esther Debbie Ismailoff, Senior Copy Editor Ita Olesker, Senior Copy Editor Commentary by Chava Boylan Rabbi Adin Even-Israel Steinsaltz Suri Brand Ilana Brown Koren Publishers Jerusalem Ltd. Carolyn Budow Ben-David POB 4044, Jerusalem 91040, ISRAEL Rachelle Emanuel POB 8531, New Milford, CT 06776, USA Charmaine Gruber Deborah Meghnagi Bailey www.korenpub.com Deena Nataf Dvora Rhein All rights reserved to Adin Steinsaltz © 2015, 2019 Elisheva Ruffer First edition 2019 Ilana Sobel Koren Tanakh Font © 1962, 2019 Koren Publishers Jerusalem Ltd. Maps Editors Koren Siddur Font and text design © 1981, 2019 Koren Publishers Jerusalem Ltd. Ilana Sobel, Map Curator Steinsaltz Center is the parent organization Rabbi Dr. Joshua Amaru, Senior Map Editor of institutions established by Rabbi Adin Even-Israel Steinsaltz Rabbi Alan Haber POB 45187, Jerusalem 91450 ISRAEL Rabbi Aryeh Sklar Telephone: +972 2 646 0900, Fax +972 2 624 9454 www.steinsaltz-center.org Language Experts Dr. Stéphanie E. Binder, Greek & Latin Considerable research and expense have gone into the creation of this publication. Rabbi Yaakov Hoffman, Arabic Unauthorized copying may be considered geneivat da’at and breach of copyright law. Dr. Shai Secunda, Persian No part of this publication (content or design, including use of the Koren fonts) may Shira Shmidman, Aramaic be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying or otherwise, without the prior written permission of the publisher, except in the case of brief quotations embedded in critical articles or reviews. -

Nitrobenzene
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization or the World Health Organization. Environmental Health Criteria 230 NITROBENZENE First draft prepared by L. Davies, Office of Chemical Safety, Therapeutic Goods Administration, Australian Department of Health and Ageing, Canberra, Australia Plese note that the pagination and layout of this web verson are not identical to those of the (to be) printed document Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2003 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO) and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. -

The Mystery, Meaning and Disappearance of the Tekhelet
THE MYSTERY, MEANING AND DISAPPEARANCE OF THE TEKHELET YOSEF GREEN AND PINCHAS KAHN And the Lord spoke to Moses, saying , "Speak to the people of Israel, and bid them that they make them fringes [tzitzit ] on the borders of their garments throughout their generations, and that they put upon the fringe of the borders a thread of blue [Petil Tekhelet]. And it shall be to you for a fringe, that you may look upon it, and remem- ber all the commandments of the Lord, and do them; and that you seek not after your own heart and your own eyes, which incline you to go astray ; That you may remember, and do all my command- 1 ments, and be holy to your God" (Num. 15:37-40). This text is climaxed by the commands to look upon the fringes, to remem- ber the commandments, and to do them. But, why and how do the fringes enable a person to become holy to your God ? The rabbinic interpretation and explication of these biblical verses and the disappearance of the tekhelet fringe will lead us to a rather surprising exposi- tion of talmudic mysticism. Our inquiry begins with a quote from T.B. Menahot 43b. The Talmud there first quotes from the biblical text in a Tannaitic source ( Baraita ), and then adds a critical point: "That you may look upon it and remember . and do them . Looking [upon it] leads to remembering [the commandments], and re- membering leads to doing them." This comment presumably relates to the issue of looking at the fringe in order to remember the commandments. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Synthesis of 2, 4-Dinitrophenoxyacetic Acid, Pyridylglycine and 2- Methoxy-5-Nitrophenylglycine As Intermediates for Indigo Dyes
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.Volume 9, Issue 2 Ver. II (Feb. 2016), PP 01-05 www.iosrjournals.org Synthesis of 2, 4-Dinitrophenoxyacetic Acid, Pyridylglycine and 2- Methoxy-5-Nitrophenylglycine as Intermediates for Indigo Dyes Nwokonkwo, D.C1, Nwokonkwo, H.C2, Okpara, N. E3 ,2,3 1Faculty of Science Industrial Chemistry Department Ebonyi State University Abakaliki, Nigeria Abstract : The preparation of indigo dye intermediates was carried out using 2, 4-dinitrophenol, 2- aminopyridine and 2-methoxy-5-nitroaniline as starting materials or reactants in the search for new indigo dye intermediates. Approximately 0.5 mole of 2, 4-dinitrophenol, 0.42 mole of 2- aminopyridine and 0.2 mole of 2- methoxy-5-nitroaniline, were treated separately with appropriate quantity of chloroacetic acid and sodium hydroxide pellets using nitrobenzene a high boiling liquid as solvent. The products that resulted: 2, 4- dinitrophenoxyacetic acid, pyridylglycine and 2-methoxy-5-nitrophenylglycine were purified using solvent extraction, activated carbon and column chromatography to reveal different amorphous powders in each case. Their structures were established by spectral analysis: ultraviolet spectroscopy (UV), infrared spectroscopy (IR), mass spectrometry (MS), proton nuclear magnetic resonance spectroscopy (1H-NMR) and carbon- 13 magnetic resonance spectroscopy (13C-NMR). Keywords: Analysis, Dye, Extraction, Intermediates, Precursors, Solvent I. Introduction The sources of organic raw materials for the synthetic dyes are mainly coal tar distillate from petroleum industry. These primary raw materials are benzene, toluene, o-, m- and p- xylenes naphthalenes anthracene etc [1]. A great variety of inorganic chemicals are also used as well. In some cases, laboratory preparation of the required compound may act as the sources of the raw material. -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -
Color Vision Light
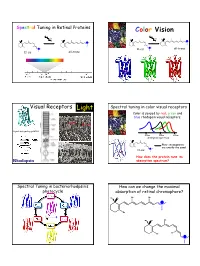
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Investigation of Nitro-Organic Compounds in Diesel Engine Exhaust: DE-AC36-08-GO28308 Final Report, February 2007–April 2008 5B
Subcontract Report Investigation of Nitro-Organic NREL/SR-540-45597 Compounds in Diesel Engine June 2010 Exhaust Final Report February 2007 – April 2008 John Dane and Kent J. Voorhees Colorado School of Mines Department of Chemistry and Geochemistry Golden, Colorado Subcontract Report Investigation of Nitro-Organic NREL/SR-540-45597 Compounds in Diesel Engine June 2010 Exhaust Final Report February 2007 – April 2008 John Dane and Kent J. Voorhees Colorado School of Mines Department of Chemistry and Geochemistry Golden, Colorado NREL Technical Monitor: Matthew Ratcliff Prepared under Subcontract No. NEV-7-77395-01 National Renewable Energy Laboratory 1617 Cole Boulevard, Golden, Colorado 80401-3393 303-275-3000 • www.nrel.gov NREL is a national laboratory of the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Operated by the Alliance for Sustainable Energy, LLC Contract No. DE-AC36-08-GO28308 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof.