Hesperia Colorado Oregonia, Oregon Branded
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Self-Repair and Self-Cleaning of the Lepidopteran Proboscis
Clemson University TigerPrints All Dissertations Dissertations 8-2019 Self-Repair and Self-Cleaning of the Lepidopteran Proboscis Suellen Floyd Pometto Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Pometto, Suellen Floyd, "Self-Repair and Self-Cleaning of the Lepidopteran Proboscis" (2019). All Dissertations. 2452. https://tigerprints.clemson.edu/all_dissertations/2452 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. SELF-REPAIR AND SELF-CLEANING OF THE LEPIDOPTERAN PROBOSCIS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy ENTOMOLOGY by Suellen Floyd Pometto August 2019 Accepted by: Dr. Peter H. Adler, Major Advisor and Committee Co-Chair Dr. Eric Benson, Committee Co-Chair Dr. Richard Blob Dr. Patrick Gerard i ABSTRACT The proboscis of butterflies and moths is a key innovation contributing to the high diversity of the order Lepidoptera. In addition to taking nectar from angiosperm sources, many species take up fluids from overripe or sound fruit, plant sap, animal dung, and moist soil. The proboscis is assembled after eclosion of the adult from the pupa by linking together two elongate galeae to form one tube with a single food canal. How do lepidopterans maintain the integrity and function of the proboscis while foraging from various substrates? The research questions included whether lepidopteran species are capable of total self- repair, how widespread the capability of self-repair is within the order, and whether the repaired proboscis is functional. -

Pollinator Butterfly Habitat
The ecology and conservation of grassland butterflies in the central U.S. Dr. Ray Moranz Moranz Biological Consulting 4514 North Davis Court Stillwater, Oklahoma 74075 Outline of the Presentation, Part I • Basic butterfly biology • Butterflies as pollinators • Rare butterflies of Kansas Outline of the Presentation, Part 2 • Effects of fire and grazing on grassland butterflies • Resources to learn more about butterflies • 15 common KS butterflies Life Cycle of a Painted Lady, Vanessa cardui Egg Larva Adult Chrysalis Some butterflies migrate The Monarch is the best-known migratory butterfly Knife River Indian Villages National Historic Site, North Dakota Fall migratory pathways of the Monarch The Painted Lady is another migrant Kirtland Air Force Base, New Mexico Other butterflies are non- migratory Such as this regal fritillary, seen in Anderson County, Kansas Implications of migratory status -migratory butterflies aren’t vulnerable to prescribed burns in winter and early spring (they haven’t arrived yet) -full-year resident butterflies ARE vulnerable to winter and spring fires -migratory butterflies may need lots of nectar sources on their flyway to fuel their flight Most butterfly caterpillars are host plant specialists Implications of host plant specialization • If you have the host plant, you probably have the butterfly • If you plant their host, the butterfly may follow • If you and your neighbors lack the host plants, you are unlikely to see the butterflies except during migration Butterflies as pollinators • Bees pollinate more plant -

List of Animal Species with Ranks October 2017
Washington Natural Heritage Program List of Animal Species with Ranks October 2017 The following list of animals known from Washington is complete for resident and transient vertebrates and several groups of invertebrates, including odonates, branchipods, tiger beetles, butterflies, gastropods, freshwater bivalves and bumble bees. Some species from other groups are included, especially where there are conservation concerns. Among these are the Palouse giant earthworm, a few moths and some of our mayflies and grasshoppers. Currently 857 vertebrate and 1,100 invertebrate taxa are included. Conservation status, in the form of range-wide, national and state ranks are assigned to each taxon. Information on species range and distribution, number of individuals, population trends and threats is collected into a ranking form, analyzed, and used to assign ranks. Ranks are updated periodically, as new information is collected. We welcome new information for any species on our list. Common Name Scientific Name Class Global Rank State Rank State Status Federal Status Northwestern Salamander Ambystoma gracile Amphibia G5 S5 Long-toed Salamander Ambystoma macrodactylum Amphibia G5 S5 Tiger Salamander Ambystoma tigrinum Amphibia G5 S3 Ensatina Ensatina eschscholtzii Amphibia G5 S5 Dunn's Salamander Plethodon dunni Amphibia G4 S3 C Larch Mountain Salamander Plethodon larselli Amphibia G3 S3 S Van Dyke's Salamander Plethodon vandykei Amphibia G3 S3 C Western Red-backed Salamander Plethodon vehiculum Amphibia G5 S5 Rough-skinned Newt Taricha granulosa -

Burke from Home Home
BURKE FROM HOME HOME ALL AGES! BEST FOR GRADES 2-8 LAND ACKNOWLEDGEMENT The Burke Museum stands on the lands of the Coast Salish peoples, whose ancestors resided here since time immemorial. Many Indigenous peoples thrive in this place—alive and strong. WHAT’S INSIDE • Learn about two important Indigenous building styles in the Pacific Northwest and how environment influences engineering. • Animals build homes too! Go for a walk to look for signs of animal neighbors, then try your hand at helping a crafty creature decorate its home. • Reflect on what “home” means to you and design an object label for something that is meaningful in your own home. INTRODUCTION The structure of a house and the items within tell a story about the people who live there. Many Tribes across the United States build traditional homes called longhouses. The materials used and the overall structure varies, depending on the environment and resources where the Tribe is from, but regardless, the longhouse is the center and heart of the people. In the Pacific Northwest, the longhouse has many names: longhouse, cedar plank house, smoke house and lodge. Traditionally these buildings would house multiple families and be a place for social and ceremonial gatherings, including, hosting neighboring Tribes. Today, Tribes throughout the Pacific Northwest continue to gather in community centers built like these traditional homes. Both traditional and contemporary methods and materials are used to construct them. These buildings are examples of living traditions! The Community Building at Celilo Village east of The Dalles, Oregon Many people have the misconception that tipis are is modeled after a traditional tule mat longhouse. -

Campbell Et Al. 2020 Peerj.Pdf
A novel curation system to facilitate data integration across regional citizen science survey programs Dana L. Campbell1, Anne E. Thessen2,3 and Leslie Ries4 1 Division of Biological Sciences, School of STEM, University of Washington, Bothell, WA, USA 2 The Ronin Institute for Independent Scholarship, Montclair, NJ, USA 3 Center for Genome Research and Biocomputing, Oregon State University, Corvallis, OR, USA 4 Department of Biology, Georgetown University, Washington, DC, USA ABSTRACT Integrative modeling methods can now enable macrosystem-level understandings of biodiversity patterns, such as range changes resulting from shifts in climate or land use, by aggregating species-level data across multiple monitoring sources. This requires ensuring that taxon interpretations match up across different sources. While encouraging checklist standardization is certainly an option, coercing programs to change species lists they have used consistently for decades is rarely successful. Here we demonstrate a novel approach for tracking equivalent names and concepts, applied to a network of 10 regional programs that use the same protocols (so-called “Pollard walks”) to monitor butterflies across America north of Mexico. Our system involves, for each monitoring program, associating the taxonomic authority (in this case one of three North American butterfly fauna treatments: Pelham, 2014; North American Butterfly Association, Inc., 2016; Opler & Warren, 2003) that shares the most similar overall taxonomic interpretation to the program’s working species list. This allows us to define each term on each program’s list in the context of the appropriate authority’s species concept and curate the term alongside its authoritative concept. We then aligned the names representing equivalent taxonomic Submitted 30 July 2019 concepts among the three authorities. -
Mardon Skipper Site Management Plans
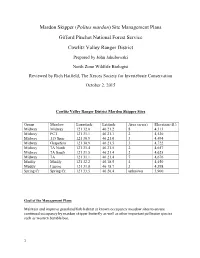
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

The Life History and Ecology of Hesperia Nabokovi in the Dominican Republic (Lepidoptera: Hesperiidae)
Vol. 1 No. 2 1990 Hesperia nabokovi: EMMEL and EMMEL 77 TROPICAL LEPIDOPTERA, 1(2): 77-82 THE LIFE HISTORY AND ECOLOGY OF HESPERIA NABOKOVI IN THE DOMINICAN REPUBLIC (LEPIDOPTERA: HESPERIIDAE) THOMAS C. EMMEL and JOHN F. EMMEL Department of Zoology, University of Florida, Gainesville, FL 32611, and 720 East Latham Avenue, Hemet, CA 92343, USA ABSTRACT.— The life history and ecology of Hesperia nabokovi (Bell & Comstock), the only species of this genus outside the Holarctic Region, is described; egg, larval and pupal characters confirm its generic assignment. The complete life cycle takes 94 days. KEY WORDS: Atalopedes, Calisto, Haiti, Gramineae, Hesperia comma, Hispaniola, immature stages, Nymphalidae, Oarisma stillmani, Satyrinae. to collect living females in the Dominican Republic, confine one of these and obtain an egg which was reared through by the second co-author (JFE) in California on a substitute grass foodplant. The purpose of this paper is to describe the elements of the life history and present some ecological notes on the biology of this fascinating Caribbean member of the genus Hesperia. DISTRIBUTION AND HABITAT The known distribution of Hesperia nabokovi is restricted to three general regions on the island of Hispaniola (Schwartz, 1989). The three areas are widely separated geographically and arc divided topographically from each other by high mountain ridges. These regions are; 1) the Cul de Sac-Valle de Neiba plain and the extreme western edge of the Llanos de Azua, from Fig. 1. Habitat for Hesperia nabokovi adults, 1km SE Monte Cristi (50ft elev.), Thomazeau and Fon Parisien in Haiti in the west to Canoa in the near the northern Dominican Republic coast, (photo by T. -

Two New Records for the Appalachian Grizzled Skipper (Pyrgus Wyandot)
Banisteria, Number 24, 2004 © 2004 by the Virginia Natural History Society Status of the Appalachian Grizzled Skipper (Pyrgus centaureae wyandot) in Virginia Anne C. Chazal, Steven M. Roble, Christopher S. Hobson, and Katharine L. Derge1 Virginia Department of Conservation and Recreation Division of Natural Heritage 217 Governor Street Richmond, Virginia 23219 ABSTRACT The Appalachian grizzled skipper (Pyrgus centaureae wyandot) was documented historically (primarily from shale barren habitats) in 11 counties in Virginia. Between 1992 and 2002, staff of the Virginia Department of Conservation and Recreation, Division of Natural Heritage, conducted 175 surveys for P. c. wyandot at 75 sites in 12 counties. The species was observed at only six sites during these surveys, representing two new county records. All observations since 1992 combined account for <80 individuals. Due to forest succession and threats from gypsy moth control measures, all recent sites for P. c. wyandot in Virginia may be degrading in overall habitat quality. Key words: Lepidoptera, Pyrgus centaureae wyandot, conservation, shale barrens, Virginia. INTRODUCTION wyandot) in Virginia. Parshall (2002) provides a comprehensive review of the nomenclature and The Appalachian grizzled skipper (Pyrgus taxonomy of P. c. wyandot. Most authors classify this centaureae wyandot) has a rather fragmented range, skipper as a subspecies of the Holarctic Pyrgus occurring in northern Michigan as well as portions of centaureae (e.g., Opler & Krizek, 1984; Iftner et al., Ohio, Pennsylvania, Maryland, West Virginia, and 1992; Shuey, 1994; Allen, 1997; Opler, 1998; Virginia; isolated historical records are known from Glassberg, 1999; Parshall, 2002), although some Kentucky, New York, New Jersey, North Carolina, and lepidopterists treat it as a full species (Shapiro, 1974; the District of Columbia (Opler, 1998; NatureServe, Schweitzer, 1989; Gochfeld & Burger, 1997). -

CA Checklist of Butterflies of Tulare County
Checklist of Buerflies of Tulare County hp://www.natureali.org/Tularebuerflychecklist.htm Tulare County Buerfly Checklist Compiled by Ken Davenport & designed by Alison Sheehey Swallowtails (Family Papilionidae) Parnassians (Subfamily Parnassiinae) A series of simple checklists Clodius Parnassian Parnassius clodius for use in the field Sierra Nevada Parnassian Parnassius behrii Kern Amphibian Checklist Kern Bird Checklist Swallowtails (Subfamily Papilioninae) Kern Butterfly Checklist Pipevine Swallowtail Battus philenor Tulare Butterfly Checklist Black Swallowtail Papilio polyxenes Kern Dragonfly Checklist Checklist of Exotic Animals Anise Swallowtail Papilio zelicaon (incl. nitra) introduced to Kern County Indra Swallowtail Papilio indra Kern Fish Checklist Giant Swallowtail Papilio cresphontes Kern Mammal Checklist Kern Reptile Checklist Western Tiger Swallowtail Papilio rutulus Checklist of Sensitive Species Two-tailed Swallowtail Papilio multicaudata found in Kern County Pale Swallowtail Papilio eurymedon Whites and Sulphurs (Family Pieridae) Wildflowers Whites (Subfamily Pierinae) Hodgepodge of Insect Pine White Neophasia menapia Photos Nature Ali Wild Wanderings Becker's White Pontia beckerii Spring White Pontia sisymbrii Checkered White Pontia protodice Western White Pontia occidentalis The Butterfly Digest by Cabbage White Pieris rapae Bruce Webb - A digest of butterfly discussion around Large Marble Euchloe ausonides the nation. Frontispiece: 1 of 6 12/26/10 9:26 PM Checklist of Buerflies of Tulare County hp://www.natureali.org/Tularebuerflychecklist.htm -

"Evolutionary Responses to Climate Change". In: Encyclopedia of Life
Evolutionary Responses to Advanced article Climate Change Article Contents . Introduction David K Skelly, Yale University, New Haven, Connecticut, USA . Observed Genetic Changes . Adaptations to Climate Change L Kealoha Freidenburg, Yale University, New Haven, Connecticut, USA . Changes in Selection Pressures . Rate of Evolution versus Rate of Climate Change . Extinction Risks . Future Prospects Online posting date: 15th September 2010 Biological responses to contemporary climate change are Everything from heat tolerance, body shape and size, and abundantly documented. We know that many species are water use physiology of plants is strongly related to the cli- shifting their geographic range and altering traits, mate conditions within a species range. From these obser- including the timing of critical life history events such as vations, a natural assumption would be that a great deal of research on the role of contemporary climate change in birth, flowering and diapause. We also know from com- driving evolutionary responses has taken place. Although parative studies of species found across the earth that a there has been an increasing amount of research very strong relationship exists between a species trait and the recently, in fact there is relatively little known about the links climatic conditions in which it is found. Together, these between contemporary climate change and evolution. The observations suggest that ongoing climate change may reasons for this are not hard to determine. There is abundant lead to evolutionary responses. Where examined, evo- documentation of biological responses to climate change lutionary responses have been uncovered in most cases. (Parmesan, 2006). Species distributions are moving pole- The effort needed to disentangle these genetic contri- ward, the timing of life history events are shifting to reflect butions to responses is substantial and so examples are lengthened growing seasons and traits such as body size are few. -

Native Grasses Benefit Butterflies and Moths Diane M
AFNR HORTICULTURAL SCIENCE Native Grasses Benefit Butterflies and Moths Diane M. Narem and Mary H. Meyer more than three plant families (Bernays & NATIVE GRASSES AND LEPIDOPTERA Graham 1988). Native grasses are low maintenance, drought Studies in agricultural and urban landscapes tolerant plants that provide benefits to the have shown that patches with greater landscape, including minimizing soil erosion richness of native species had higher and increasing organic matter. Native grasses richness and abundance of butterflies (Ries also provide food and shelter for numerous et al. 2001; Collinge et al. 2003) and butterfly species of butterfly and moth larvae. These and moth larvae (Burghardt et al. 2008). caterpillars use the grasses in a variety of ways. Some species feed on them by boring into the stem, mining the inside of a leaf, or IMPORTANCE OF LEPIDOPTERA building a shelter using grass leaves and silk. Lepidoptera are an important part of the ecosystem: They are an important food source for rodents, bats, birds (particularly young birds), spiders and other insects They are pollinators of wild ecosystems. Terms: Lepidoptera - Order of insects that includes moths and butterflies Dakota skipper shelter in prairie dropseed plant literature review – a scholarly paper that IMPORTANT OF NATIVE PLANTS summarizes the current knowledge of a particular topic. Native plant species support more native graminoid – herbaceous plant with a grass-like Lepidoptera species as host and food plants morphology, includes grasses, sedges, and rushes than exotic plant species. This is partially due to the host-specificity of many species richness - the number of different species Lepidoptera that have evolved to feed on represented in an ecological community, certain species, genus, or families of plants.