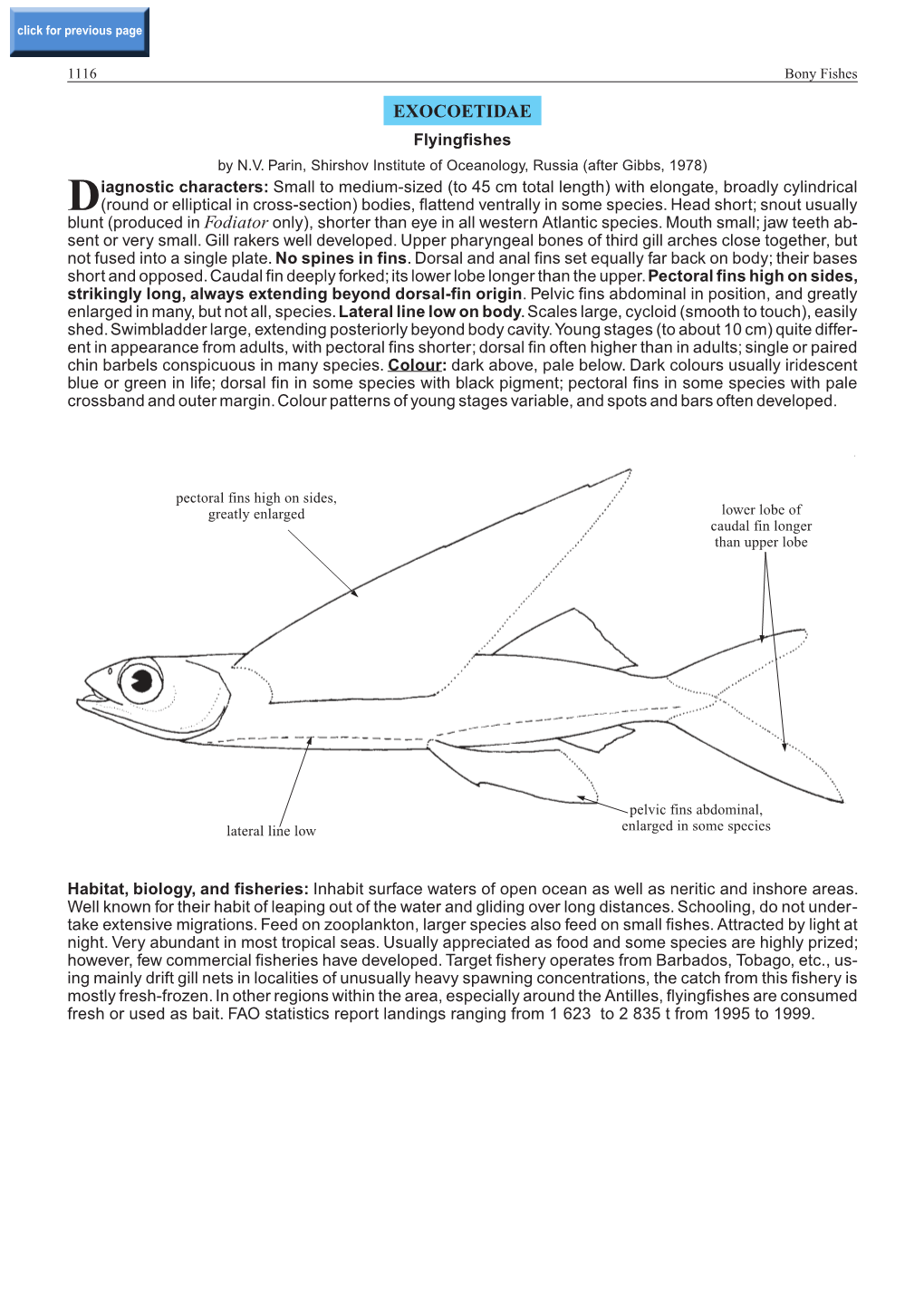
EXOCOETIDAE Flyingfishes by N.V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pacific Plate Biogeography, with Special Reference to Shorefishes
Pacific Plate Biogeography, with Special Reference to Shorefishes VICTOR G. SPRINGER m SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 367 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Appendix 1. (Online Supplementary Material) Species, Gliding Strategies
Appendix 1. (Online Supplementary Material) Species, gliding strategies, species distributions, geographic range sizes, habitat, and egg buoyancy characteristics used for concentrated changes tests. Species Gliding strategy Species distribution (reference #) Geographic range size Habitat (reference #) Egg buoyancy (reference #) Cheilopogon abei (Parin, 1996) 4 wings Indian, Indo-Pacific (1) 2 or more ocean basins meroepipelagic (1) Buoyant (2) Cheilopogon atrisignis (Jenkins, 1903) 4 wings Indian, Pacific (1) 2 or more ocean basins meroepipelgic (3) Buoyant (4) Cheilopogon cyanopterus (Valenciennes, 1847) 4 wings Atlantic, Indo-Pacific (2) 2 or more ocean basins meroepipelgic (3) Non-Buoyant (5) Cheilopogon dorsomacula (Fowler, 1944) 4 wings Pacific (1) within 1 ocean basin holoepipelagic (1) Buoyant (2) Cheilopogon exsiliens (Linnaeus, 1771) 4 wings Atlantic (2) within 1 ocean basin holoepipelagic (3) Buoyant (2,5) Cheilopogon furcatus (Mitchill, 1815) 4 wings Atlantic, Indian, Pacific (6) 2 or more ocean basins holoepipelagic (3) Non-Buoyant (5) Cheilopogon melanurus (Valenciennes, 1847) 4 wings Atlantic (7) within 1 ocean basin meroepipelagic (7) Non-Buoyant (5,8) Cheilopogon pinnatibarbatus (californicus) (Cooper, 1863) 4 wings eastern tropical Pacific (9) within 1 ocean basin meroepipelgic (3) Non-Buoyant (10) Cheilopogon spilonotopterus (Bleeker, 1865) 4 wings Indian and Pacific (1) 2 or more ocean basins meroepipelgic (3) Buoyant (4) Cheilopogon xenopterus (Gilbert, 1890) 4 wings eastern tropical Pacific (11) within 1 ocean basin -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Mediterranean Marine Science
Mediterranean Marine Science Vol. 18, 2017 “New Mediterranean Biodiversity Records” (March 2017) LIPEJ L. Marine Biology Station, National Institute of Biology, Fornače 61, 6630 Piran ACEVEDO I. Museo Nacional de Ciencias Naturales (MNCN-CSIC), José Gutiérrez Abascal, 2, 28006 Madrid AKEL E.H.K. Fishery Biology Lab, National Institute of Oceanography and Fisheries Kait-Bey, Alexandria ANASTASOPOULOU A. Hellenic Center of Marine Research, 46.7 km Athens Sounio ave., P.O. Box 712, 19013 Anavyssos ANGELIDIS A. Kapetan Vangeli 5, 54646 Thessaloniki AZZURRO E. Institute for Environmental Protection and Research (ISPRA), STS Livorno, Piazzale dei Marmi 2, 57123, Livorno CASTRIOTA L. Institute for Environmental Protection and Research, ISPRA,Via S. Puglisi 9, STS- Palermo, 90143 Palermo ÇELIK M. Faculty of Fisheries, Muğla Sıtkı Koçman University, 48000, Kötekli, Muğla CILENTI L. C.N.R. – ISMAR, UOS Lesina, Via Pola, 4 – 71010 Lesina (FG) CROCETTA F. Hellenic Center of Marine Research, 46.7 km Athens Sounio ave., P.O. Box 712, 19013 Anavyssos DEIDUN A. Department of Geosciences, University of Malta, Msida MSD 2080 DOGRAMMATZI A. Hellenic Center of Marine Research, 46.7 km Athens Sounio ave., P.O. Box 712, 19013 Anavyssos FALAUTANO M. Institute for Environmental http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 21/02/2020 06:27:07 | Protection and Research, ISPRA,Via S. Puglisi 9, STS- Palermo, 90143 Palermo FERNÁNDEZ-ÁLVAREZ Institut de Ciències del Mar F.Á. (CSIC), Passeig Maritim 37–49, 08003 Barcelona GENNAIO R. Regional Agency for Environmental Protection (ARPA) Puglia, Lecce Department, Via Miglietta 1, 73100, Lecce INSACCO G. Museo Civico di Storia Naturale, via degli Studi 9, 97013 Comiso (RG) KATSANEVAKIS S. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Diet and Stable Isotope Analyses Reveal The
RESEARCH ARTICLE Diet and stable isotope analyses reveal the feeding ecology of the orangeback squid Sthenoteuthis pteropus (Steenstrup 1855) (Mollusca, Ommastrephidae) in the eastern tropical Atlantic VeÂronique Merten1*, Bernd Christiansen2, Jamileh Javidpour1, Uwe Piatkowski1, Oscar Puebla1,3, Rebeca Gasca4, Henk-Jan T. Hoving1 a1111111111 a1111111111 1 GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany, 2 UniversitaÈt Hamburg, Institute for Hydrobiology and Fishery Sciences, Hamburg, Germany, 3 Christian-Albrechts-UniversitaÈt zu Kiel, Kiel, a1111111111 Germany, 4 El Colegio de la Frontera Sur, Chetumal, Mexico a1111111111 a1111111111 * [email protected] Abstract OPEN ACCESS In the eastern tropical Atlantic, the orangeback flying squid Sthenoteuthis pteropus Citation: Merten V, Christiansen B, Javidpour J, (Steenstrup 1855) (Cephalopoda, Ommastrephidae) is a dominant species of the epipelagic Piatkowski U, Puebla O, Gasca R, et al. (2017) Diet nekton community. This carnivore squid has a short lifespan and is one of the fastest-grow- and stable isotope analyses reveal the feeding ecology of the orangeback squid Sthenoteuthis ing squids. In this study, we characterise the role of S. pteropus in the pelagic food web of pteropus (Steenstrup 1855) (Mollusca, the eastern tropical Atlantic by investigating its diet and the dynamics of its feeding habits Ommastrephidae) in the eastern tropical Atlantic. throughout its ontogeny and migration. During three expeditions in the eastern tropical PLoS ONE 12(12): e0189691. https://doi.org/ 10.1371/journal.pone.0189691 Atlantic in 2015, 129 specimens were caught by hand jigging. Stomach content analyses (via visual identification and DNA barcoding) were combined with stable isotope data (@15N Editor: Erik V. Thuesen, Evergreen State College, 13 UNITED STATES and @ C) of muscle tissue to describe diet, feeding habits and trophic ecology of S. -

The Red-Tailed Tropicbird on Kure Atoll
THE RED-TAILED TROPICBIRD ON KURE ATOLL BY ROBERT R FLEET ORNITHOLOGICAL MONOGRAPHS NO. 16 PUBLISHED BY THE AMERICAN ORNITHOLOGISTS' UNION 1974 THE RED-TAILED TROPICBIRD ON KURE ATOLL ORNITHOLOGICAL MONOGRAPHS This series,published by the American Ornithologists'Union, has been establishedfor major paperstoo long for inclusionin the Union's journal, The Auk. Publicationhas been made possiblethrough the generosityof Mrs. Carll Tucker and the Marcia Brady Tucker Foundation,Inc. Correspondenceconcerning manuscripts for publicationin the seriesshotfid be addressedto the Editor, Dr. JohnWilliam Hardy, Florida StateMuseum, Universityof Florida, Gainesville,Florida 32611. Copies of OrnithologicalMonographs may be ordered from the Asst. Treasurerof the AOU, Glen E. Woolfenden,Dept. of Biology,University of SouthFlorida, Tampa, Florida 33620. OrnithologicalMonographs, No. 16, vi + 64 pp. Editor-in-chief, John William Hardy SpecialAssociate Editor for this issue: ThomasR. Howell Author's address:Department of Entomology,Texas A&M University, College Station, Texas 77843. Issued December 26, 1974 Price $5.50 prepaid ($4.50 to AOU Members) Library of CongressCatalogue Card Number 74-32550 Printed by the Allen Press,Inc., Lawrence,Kansas 66044 Copyright ¸ by American Ornithologists'Union, 1974 THE RED-TAILED TROPICBIRD ON KURE ATOLL BY ROBERT R. FLEET ORNITHOLOGICAL MONOGRAPHS NO. 16 PUBLISHED BY THE AMERICAN ORNITHOLOGISTS' UNION 1974 TABLE OF CONTENTS INTRODUCTION .......................................................................... 1 Locationand -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

APPENDIX 1 Classified List of Fishes Mentioned in the Text, with Scientific and Common Names
APPENDIX 1 Classified list of fishes mentioned in the text, with scientific and common names. ___________________________________________________________ Scientific names and classification are from Nelson (1994). Families are listed in the same order as in Nelson (1994), with species names following in alphabetical order. The common names of British fishes mostly follow Wheeler (1978). Common names of foreign fishes are taken from Froese & Pauly (2002). Species in square brackets are referred to in the text but are not found in British waters. Fishes restricted to fresh water are shown in bold type. Fishes ranging from fresh water through brackish water to the sea are underlined; this category includes diadromous fishes that regularly migrate between marine and freshwater environments, spawning either in the sea (catadromous fishes) or in fresh water (anadromous fishes). Not indicated are marine or freshwater fishes that occasionally venture into brackish water. Superclass Agnatha (jawless fishes) Class Myxini (hagfishes)1 Order Myxiniformes Family Myxinidae Myxine glutinosa, hagfish Class Cephalaspidomorphi (lampreys)1 Order Petromyzontiformes Family Petromyzontidae [Ichthyomyzon bdellium, Ohio lamprey] Lampetra fluviatilis, lampern, river lamprey Lampetra planeri, brook lamprey [Lampetra tridentata, Pacific lamprey] Lethenteron camtschaticum, Arctic lamprey] [Lethenteron zanandreai, Po brook lamprey] Petromyzon marinus, lamprey Superclass Gnathostomata (fishes with jaws) Grade Chondrichthiomorphi Class Chondrichthyes (cartilaginous -

Predator-Driven Macroevolution in Flyingfishes Inferred from Behavioural Studies 59
Predator-driven macroevolution in flyingfishes inferred from behavioural studies 59 Predator-driven macroevolution in flyingfishes inferred from behavioural studies: historical controversies and a hypothesis U. Kutschera Abstract Flyingfishes (Exocoetidae) are unique oceanic animals that use their tail and their large, wing-like pectoral fins to launch themselves out of the water and glide through the air. Independent observations document that flyingfishes use their gliding ability to escape from aquatic predators such as dolphins (marine mammals). The fossil record of flyingfishes is very poor. Nevertheless, the evolution of gliding among flyingfishes and their allies (Beloniformes) was analysed and reconstructed by the ethologist Konrad Lorenz (1903 – 1989) and other zoologists. In this article I review the comparative method in evolutionary biology, describe historical controversies concerning the biology and systematics of flyingfishes and present a hypothesis on the phylogenetic development of gliding among these marine vertebrates. This integrative model is based on behavioural studies and has been corroborated by molecular data (evolutionary trees derived from DNA sequences). Introduction Since the publication of Darwin´s classical book (1872, 1st ed. 1859), evolutionary biology has relied primarily upon comparative studies of extant organisms (animals, plants), supplemented whenever possible by information obtained from the fossil record. This interaction between neontological and palaeontological research has greatly enriched our knowledge of the evolutionary history (phylogeny) of a variety of macro- organisms, notably hard-shelled marine invertebrates (molluscs etc.) and vertebrates, for which thousands of well-preserved fossils have been described. Such comparative studies have become considerably more significant with the development of molecular methods for reconstructing DNA-sequence-based phylogenies and with the increased rigour with which the comparative method has been applied.