Plastic Surgery of the Ear
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CASE REPORT Resolution of Delayed Sudden Sensorineural Hearing Loss After Stapedectomy
The Mediterranean Journal of Otology CASE REPORT Resolution of Delayed Sudden Sensorineural Hearing Loss After Stapedectomy: A Case Report and Review of the Literature Noam Yehudai, MD, Michal Luntz, MD From the Department of Significant sensorineural hearing loss may develop immediately after suc- Otolaryngology, Head and Neck cessful stapedectomy but sometimes occurs months or even years later. Surgery, Bnai-Zion Medical The rate of recovery from that disorder has not been determined. Several Center, Technion-Israel School of Technology, Haifa, Israel reports in the 1960s described patients with delayed sensorineural hear- ing loss, but that entity has not been mentioned in the English-language Correspondence literature for the last 30 years. We present a review of the literature on this Michal Luntz, MD postsurgical auditory complication and describe a patient with delayed Department of Otolaryngology, Head and Neck Surgery poststapedectomy sensorineural hearing loss that developed 15 months Bnai-Zion Medical Center, after surgery and resolved completely after treatment with an oral steroid. Technion-Israel School of Technology 47 Golomb St, PO Box 4940, Haifa 31048, Israel Phone: 972-4-8359544 Fax: 972-4-8361069 E-mail: [email protected] Submitted: 05 February, 2006 Revised: 07 May, 2006 Accepted: 09 May, 2006 Mediterr J Otol 2006; 3: 156-160 Copyright 2005 © The Mediterranean Society of Otology and Audiology 156 Resolution of Delayed Sudden Sensorineural Hearing Loss After Stapedectomy: A Case Report and Review of the Literature -

Sound and the Ear Chapter 2
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Chapter© Jones & Bartlett 2 Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Sound and the Ear © Jones Karen &J. Kushla,Bartlett ScD, Learning, CCC-A, FAAA LLC © Jones & Bartlett Learning, LLC Lecturer NOT School FOR of SALE Communication OR DISTRIBUTION Disorders and Deafness NOT FOR SALE OR DISTRIBUTION Kean University © Jones & Bartlett Key Learning, Terms LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR Acceleration DISTRIBUTION Incus NOT FOR SALE OR Saccule DISTRIBUTION Acoustics Inertia Scala media Auditory labyrinth Inner hair cells Scala tympani Basilar membrane Linear scale Scala vestibuli Bel Logarithmic scale Semicircular canals Boyle’s law Malleus Sensorineural hearing loss Broca’s area © Jones & Bartlett Mass Learning, LLC Simple harmonic© Jones motion (SHM) & Bartlett Learning, LLC Brownian motion Membranous labyrinth Sound Cochlea NOT FOR SALE OR Mixed DISTRIBUTION hearing loss Stapedius muscleNOT FOR SALE OR DISTRIBUTION Compression Organ of Corti Stapes Condensation Osseous labyrinth Tectorial membrane Conductive hearing loss Ossicular chain Tensor tympani muscle Decibel (dB) Ossicles Tonotopic organization © Jones Decibel & hearing Bartlett level (dB Learning, HL) LLC Outer ear © Jones Transducer & Bartlett Learning, LLC Decibel sensation level (dB SL) Outer hair cells Traveling wave theory NOT Decibel FOR sound SALE pressure OR level DISTRIBUTION -

Auditory Brainstem Response Latency in Noise As a Marker of Cochlear Synaptopathy
The Journal of Neuroscience, March 30, 2016 • 36(13):3755–3764 • 3755 Systems/Circuits Auditory Brainstem Response Latency in Noise as a Marker of Cochlear Synaptopathy X Golbarg Mehraei,1,2 XAnn E. Hickox,1,4 X Hari M. Bharadwaj,2,7 Hannah Goldberg,1 Sarah Verhulst,2,6 M. Charles Liberman,1,4,5 and XBarbara G. Shinn-Cunningham2,3 1Program in Speech and Hearing Bioscience and Technology, Harvard University/Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, 2Center for Computational Neuroscience and Neural Technology, Boston University, Boston, Massachusetts 02215, 3Department of Biomedical Engineering, Boston University, Boston, Massachusetts 02215, 4Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts 02114, 5Department of Otology and Laryngology, Harvard Medical School, Boston, Massachusetts 02114, 6Cluster of Excellence Hearing4All and Medical Physics, Department of Medical Physics and Acoustics, Oldenburg University, 26129 Oldenburg, Germany, 7Martinos Center for Biomedical Imaging, Department of Neurology, Massachusetts General Hospital/Harvard Medical School, Charlestown, Massachusetts 02129 Evidence from animal and human studies suggests that moderate acoustic exposure, causing only transient threshold elevation, can nonetheless cause “hidden hearing loss” that interferes with coding of suprathreshold sound. Such noise exposure destroys synaptic connections between cochlear hair cells and auditory nerve fibers; however, there is no clinical test of this synaptopathy in humans. In animals, synaptopathy reduces the amplitude of auditory brainstem response (ABR) wave-I. Unfortunately, ABR wave-I is difficult to measure in humans, limiting its clinical use. Here, using analogous measurements in humans and mice, we show that the effect of masking noise on the latency of the more robust ABR wave-V mirrors changes in ABR wave-I amplitude. -

A Pictorial Review of Round Window Pathologies
REVIEW ARTICLE The Forgotten Second Window: A Pictorial Review of Round Window Pathologies J.C. Benson, F. Diehn, T. Passe, J. Guerin, V.M. Silvera, M.L. Carlson, and J. Lane ABSTRACT SUMMARY: The round window serves to decompress acoustic energy that enters the cochlea via stapes movement against the oval window. Any inward motion of the oval window via stapes vibration leads to outward motion of the round window. Occlusion of the round window is a cause of conductive hearing loss because it increases the resistance to sound energy and con- sequently dampens energy propagation. Because the round window niche is not adequately evaluated by otoscopy and may be incompletely exposed during an operation, otologic surgeons may not always correctly identify associated pathology. Thus, radiol- ogists play an essential role in the identification and classification of diseases affecting the round window. The purpose of this review is to highlight the developmental, acquired, neoplastic, and iatrogenic range of pathologies that can be encountered in round window dysfunction. ABBREVIATIONS: LO 4 labyrinthitis ossificans; RW 4 round window he round window (RW) serves as a boundary between the considerations of the round window that can be encoun- Tbasal turn of the cochlea anteriorly and the round win- tered on imaging are reviewed. dow niche posteriorly.1 It, along with the oval window, is 1 of 2 natural openings between the inner and middle ear. The Physiology and Functionality “ ” round window is often overshadowed by the first window The inner ear “windows” refer to openings in the otic capsule “ ” (the oval window) and pathologic third windows (eg, that connect the fluid in the inner ear to either the middle ear or superior semicircular canal dehiscence). -

Hearing Loss Due to Myringotomy and Tube Placement and the Role of Preoperative Audiograms
ORIGINAL ARTICLE Hearing Loss Due to Myringotomy and Tube Placement and the Role of Preoperative Audiograms Mark Emery, MD; Peter C. Weber, MD Background: Postoperative complications of myrin- erative and postoperative sensorineural and conductive gotomy and tube placement often include otorrhea, tym- hearing loss. panosclerosis, and tympanic membrane perforation. How- ever, the incidence of sensorineural or conductive hearing Results: No patient developed a postoperative sensori- loss has not been documented. Recent efforts to curb the neural or conductive hearing loss. All patients resolved use of preoperative audiometric testing requires docu- their conductive hearing loss after myringotomy and tube mentation of this incidence. placement. There was a 1.3% incidence of preexisting sen- sorineural hearing loss. Objective: To define the incidence of conductive and sensorineural hearing loss associated with myrin- Conclusions: The incidence of sensorineural or con- gotomy and tube placement. ductive hearing loss after myringotomy and tube place- ment is negligible and the use of preoperative audiomet- Materials and Methods: A retrospective chart re- ric evaluation may be unnecessary in selected patients, view of 550 patients undergoing myringotomy and tube but further studies need to be done to corroborate this placement was performed. A total of 520 patients under- small data set. going 602 procedures (1204 ears), including myrin- gotomy and tube placement, were assessed for preop- Arch Otolaryngol Head Neck Surg. 1998;124:421-424 TITIS MEDIA (OM) is one erative hearing status and whether it has of the most frequent dis- either improved or remained stable after eases of childhood, af- MTT. A recent report by Manning et al11 fecting at least 80% of demonstrated a 1% incidence of preop- children prior to school erative sensorineural hearing loss (SNHL) Oentry.1-4 Because of the high incidence of in children undergoing MTT. -

The Round Window Region and Contiguous Areas: Endoscopic Anatomy and Surgical Implications
Eur Arch Otorhinolaryngol DOI 10.1007/s00405-014-2923-8 OTOLOGY The round window region and contiguous areas: endoscopic anatomy and surgical implications Daniele Marchioni · Matteo Alicandri-Ciufelli · David D. Pothier · Alessia Rubini · Livio Presutti Received: 16 October 2013 / Accepted: 28 January 2014 © Springer-Verlag Berlin Heidelberg 2014 Abstract The round window region is a critical area of window region. Exact anatomical knowledge of this region the middle ear; the aim of this paper is to describe its anat- can have important advantages during surgery, since some omy from an endoscopic perspective, emphasizing some pathology can invade inside cavities or tunnels otherwise structures, the knowledge of which could have important not seen by instrumentation that produces a straight-line implications during surgery, as well as to evaluate what view (e.g. microscope). involvement cholesteatoma may have with these structures. Retrospective review of video recordings of endoscopic Keywords Retrotympanum · Endoscopic ear surgery · ear surgeries and retrospective database review were con- Cholesteatoma · Middle ear anatomy · Round window ducted in Tertiary university referral center. Videos from endoscopic middle ear procedures carried out between June 2010 and September 2012 and stored in a shared data- Introduction base were reviewed retrospectively. Surgeries in which an endoscopic magnification of the round window region and The surgical management of cholesteatoma is still a contro- the inferior retrotympanum area was performed intraop- versial issue. Endoscopic instrumentation, techniques and eratively were included in the study. Involvement by cho- knowledge have improved considerably over the last few lesteatoma of those regions was also documented based years, and we believe that, in the future, endoscopic surgi- on information obtained from the surgical database. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

The Posterior Muscles of the Auricle: Anatomy and Surgical Applications
Central Annals of Otolaryngology and Rhinology Research Article *Corresponding author Christian Vacher, Department of Maxillofacial Surgery & Anatomy, University of Paris-Diderot, APHP, 100, The Posterior Muscles of the Boulevard Général Leclerc, 92110 Clichy, France, Tel: 0033140875671; Email: Submitted: 19 December 2014 Auricle: Anatomy and Surgical Accepted: 16 January 2015 Published: 19 January 2015 Applications Copyright © 2015 Vacher et al. Rivka Bendrihem1, Christian Vacher2* and Jacques Patrick Barbet3 OPEN ACCESS 1 Department of Dentistry, University of Paris-Descartes, France Keywords 2 Department of Maxillofacial Surgery & Anatomy, University of Paris-Diderot, France • Auricle 3 Department of Pathology and Cytology, University of Paris-Descartes, France • Anatomy • Prominent ears Abstract • Muscle Objective: Prominent ears are generally considered as primary cartilage deformities, but some authors consider that posterior auricular muscles malposition could play a role in the genesis of this malformation. Study design: Auricle dissections of 30 cadavers and histologic sections of 2 fetuses’ ears. Methods: Posterior area of the auricle has been dissected in 24 cadavers preserved with zinc chlorure and 6 fresh cadavers in order to describe the posterior muscles and fascias of the auricle. Posterior auricle muscles from 5 fresh adult cadavers have been performed and two fetal auricles (12 and 22 weeks of amenorhea) have been semi-serially sectioned in horizontal plans. Five µm-thick sections were processed for routine histology (H&E) or for immuno histochemistry using antibodies specific for the slow-twitch and fast-twich myosin heavy chains in order to determine which was the nature of these muscles. Results: The posterior auricular and the transversus auriculae muscles looked in most cases like skeletal muscles and they were made of 75% of slow muscular fibres. -

Anatomical Changes and Audiological Profile in Branchio-Oto-Renal
THIEME 68 Review Article Anatomical Changes and Audiological Profile in Branchio-oto-renal Syndrome: A Literature Review Tâmara Andrade Lindau1 Ana Cláudia Vieira Cardoso1 Natalia Freitas Rossi1 Célia Maria Giacheti1 1 Department of Speech Pathology, Universidade Estadual Paulista - Address for correspondence Célia Maria Giacheti, PhD, Department of UNESP, Marília, São Paulo, Brazil Speech Pathology, Universidade Estadual Paulista UNESP, Av. Hygino Muzzi Filho, 737, Marília, São Paulo 14525-900, Brazil Int Arch Otorhinolaryngol 2014;18:68–76. (e-mail: [email protected]). Abstract Introduction Branchio-oto-renal (BOR) syndrome is an autosomal-dominant genetic condition with high penetrance and variable expressivity, with an estimated prevalence of 1 in 40,000. Approximately 40% of the patients with the syndrome have mutations in the gene EYA1, located at chromosomal region 8q13.3, and 5% have mutations in the gene SIX5 in chromosome region 19q13. The phenotype of this syndrome is character- ized by preauricular fistulas; structural malformations of the external, middle, and inner ears; branchial fistulas; renal disorders; cleft palate; and variable type and degree of hearing loss. Aim Hearing loss is part of BOR syndrome phenotype. The aim of this study was to present a literature review on the anatomical aspects and audiological profile of BOR syndrome. Keywords Data Synthesis Thirty-four studies were selected for analysis. Some aspects when ► branchio-oto-renal specifying the phenotype of BOR syndrome are controversial, especially those issues syndrome related to the audiological profile in which there was variability on auditory standard, ► BOR syndrome hearing loss progression, and type and degree of the hearing loss. -

Instruction Sheet: Otitis Externa
University of North Carolina Wilmington Abrons Student Health Center INSTRUCTION SHEET: OTITIS EXTERNA The Student Health Provider has diagnosed otitis externa, also known as external ear infection, or swimmer's ear. Otitis externa is a bacterial/fungal infection in the ear canal (the ear canal goes from the outside opening of the ear to the eardrum). Water in the ear, from swimming or bathing, makes the ear canal prone to infection. Hot and humid weather also predisposes to infection. Symptoms of otitis externa include: ear pain, fullness or itching in the ear, ear drainage, and temporary loss of hearing. These symptoms are similar to those caused by otitis media (middle ear infection). To differentiate between external ear infection and middle ear infection, the provider looks in the ear with an instrument called an otoscope. It is important to distinguish between the two infections, as they are treated differently: External otitis is treated with drops in the ear canal, while middle ear infection is sometimes treated with an antibiotic by mouth. MEASURES YOU SHOULD TAKE TO HELP TREAT EXTERNAL EAR INFECTION: 1. Use the ear drops regularly, as directed on the prescription. 2. The key to treatment is getting the drops down into the canal and keeping the medicine there. To accomplish this: Lie on your side, with the unaffected ear down. Put three to four drops in the infected ear canal, then gently pull the outer ear back and forth several times, working the medicine deeper into the ear canal. Remain still, good-ear-side-down for about 15 minutes. -
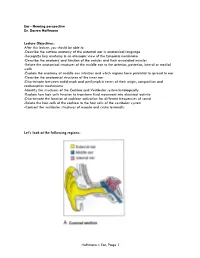
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Bedside Neuro-Otological Examination and Interpretation of Commonly
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.054478 on 24 November 2004. Downloaded from BEDSIDE NEURO-OTOLOGICAL EXAMINATION AND INTERPRETATION iv32 OF COMMONLY USED INVESTIGATIONS RDavies J Neurol Neurosurg Psychiatry 2004;75(Suppl IV):iv32–iv44. doi: 10.1136/jnnp.2004.054478 he assessment of the patient with a neuro-otological problem is not a complex task if approached in a logical manner. It is best addressed by taking a comprehensive history, by a Tphysical examination that is directed towards detecting abnormalities of eye movements and abnormalities of gait, and also towards identifying any associated otological or neurological problems. This examination needs to be mindful of the factors that can compromise the value of the signs elicited, and the range of investigative techniques available. The majority of patients that present with neuro-otological symptoms do not have a space occupying lesion and the over reliance on imaging techniques is likely to miss more common conditions, such as benign paroxysmal positional vertigo (BPPV), or the failure to compensate following an acute unilateral labyrinthine event. The role of the neuro-otologist is to identify the site of the lesion, gather information that may lead to an aetiological diagnosis, and from there, to formulate a management plan. c BACKGROUND Balance is maintained through the integration at the brainstem level of information from the vestibular end organs, and the visual and proprioceptive sensory modalities. This processing takes place in the vestibular nuclei, with modulating influences from higher centres including the cerebellum, the extrapyramidal system, the cerebral cortex, and the contiguous reticular formation (fig 1).