Public Release Summary On
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects
EN58CH06-Casida ARI 5 December 2012 8:11 Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects John E. Casida1,∗ and Kathleen A. Durkin2 1Environmental Chemistry and Toxicology Laboratory, Department of Environmental Science, Policy, and Management, 2Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, California 94720; email: [email protected], [email protected] Annu. Rev. Entomol. 2013. 58:99–117 Keywords The Annual Review of Entomology is online at acetylcholinesterase, calcium channels, GABAA receptor, nicotinic ento.annualreviews.org receptor, secondary targets, sodium channel This article’s doi: 10.1146/annurev-ento-120811-153645 Abstract Copyright c 2013 by Annual Reviews. Neuroactive insecticides are the principal means of protecting crops, people, All rights reserved livestock, and pets from pest insect attack and disease transmission. Cur- ∗ Corresponding author rently, the four major nerve targets are acetylcholinesterase for organophos- phates and methylcarbamates, the nicotinic acetylcholine receptor for neonicotinoids, the γ-aminobutyric acid receptor/chloride channel for by Public Health Information Access Project on 04/29/14. For personal use only. Annu. Rev. Entomol. 2013.58:99-117. Downloaded from www.annualreviews.org polychlorocyclohexanes and fiproles, and the voltage-gated sodium channel for pyrethroids and dichlorodiphenyltrichloroethane. Species selectivity and acquired resistance are attributable in part to structural differences in binding subsites, receptor subunit interfaces, or transmembrane regions. Additional targets are sites in the sodium channel (indoxacarb and metaflumizone), the glutamate-gated chloride channel (avermectins), the octopamine receptor (amitraz metabolite), and the calcium-activated calcium channel (diamides). Secondary toxic effects in mammals from off-target serine hydrolase inhibi- tion include organophosphate-induced delayed neuropathy and disruption of the cannabinoid system. -

BOARD of PESTICIDES CONTROL January 15, 2020 Augusta Civic Center, 76 Community Drive, Kennebec/Penobscot Room, Augusta, Maine
STATE OF MAINE DEPARTMENT OF AGRICULTURE, CONSERVATION AND FORESTRY BOARD OF PESTICIDES CONTROL 28 STATE HOUSE STATION UGUSTA AINE JANET T. MILLS A , M 04333 AMANDA E. BEAL GOVERNOR COMMISSIONER BOARD OF PESTICIDES CONTROL January 15, 2020 Augusta Civic Center, 76 Community Drive, Kennebec/Penobscot Room, Augusta, Maine 1:00 - 1:30 PM Board Meeting 1:30 - 2:30 PM Public Forum On Notification 2:30 – 4:00 PM Board Meeting Continued AGENDA 1. Introductions of Board and Staff 2. Minutes of the November 8, 2019 Board Meeting Presentation By: Megan Patterson, Director Action Needed: Amend and/or Approve 3. Request for Financial Support from the Maine Mobile Health Program and the Eastern Maine Development Corporation Since 1995 the Board has supported a Migrant and Seasonal Farmworker Safety Education program. The Maine Mobile Health Program (MMHP) and Eastern Maine Development Corporation (EMDC provided training to 315 migrant agricultural workers during the 2019 season). Funding to support this effort in 2020 is being requested in the amount of $5,360, which is the same amount the Board provided in 2019. The funding has been accounted for in the Board’s FY20 budget. Presentation By: Chris Huh, Program Manager, Farmworkers Jobs Program, Eastern Maine Development Corporation Elizabeth Charles McGough, Director of Outreach, Maine Mobile Health Program MEGAN PATTERSON, DIRECTOR PHONE: (207) 287-2731 90 BLOSSOM LANE, DEERING BUILDING WWW.THINKFIRSTSPRAYLAST.ORG Action Needed: Discussion and Determination if the Board Wishes to Fund this Request 4. Request for Financial Support from the Maine State Apiarist for CLEAR Training Maine State Apiarist, Jennifer Lund, has requested funding to attend the National Certified Investigator & Inspector Basic Training held in Raleigh, North Carolina in March 2020. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

Impact of Insecticides Used to Control Spodoptera Frugiperda (J.E
RESEARCH Impact of insecticides used to control Spodoptera frugiperda (J.E. Smith) in corn on survival, sex ratio, and reproduction of Trichogramma pretiosum Riley offspring Jander R. Souza1, Geraldo A. Carvalho1, Alexandre P. Moura2*, Marcelo H.G. Couto1, and Jader B. Maia1 Corn (Zea mays L.) is cultivated in large areas and considered one of the world’s major cereal crops. There are several arthropod pests that can reduce its production such as the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lep.: Noctuidae), which is considered to be the main pest for corn. Fall armyworm is primarily controlled by insecticides. The use of biological control agents to manage this pest is growing with an emphasis on the egg parasitoid Trichogramma pretiosum Riley (Hym.: Trichogrammatidae). Thus, the aim of this research was to evaluate the impact of the following insecticides (g ai L-1) beta-cypermethrin (0.03), chlorfenapyr (0.60), chlorpyrifos (0.96), spinosad (0.16), etofenprox (0.10), triflumuron (0.08), alfa-cypermethrin/teflubenzuron (0.0425/0.0425), and lambda-cyhalothrin/thiamethoxam (0.11/0.083) on survival, sex ratio, reproduction, and T. pretiosum offspring. Distilled water was used as a control. Commercial insecticide formulations were diluted in distilled water. Bioassays used Anagasta kuehniella eggs treated with insecticides which were afterwards exposed to parasitism. Bioassays were conducted under controlled conditions at 25 ± 2 ºC, 70 ± 10% RH, and 12:12 h photoperiod. Alfa-cypermethrin/teflubenzuron, beta-cypermethrin, chlorpyrifos, chlorfenapyr, spinosad, etofenprox, and lambda-cyhalothrin/thiamethoxam reduced parasitism capacity of maternal generation females as well as the percentage of insect emergence from the F1 generation. -
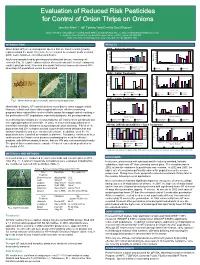
Evaluation of Reduced Risk Pesticides for Control of Onion Thrips on Onions
Evaluation of Reduced Risk Pesticides for Control of Onion Thrips on Onions Jennifer Allen1,3, Jeff Tolman2 and Cynthia Scott-Dupree3 1 – Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph ON N1G 4Y2, E-mail: [email protected] 2 – Southern Crop Protection and Food Research Centre – AAFC, London ON N5V 4T3 3 – Dept. Environmental Biology, University of Guelph, Guelph ON N1G 2W1 INTRODUCTION RESULTS Onion thrips (OT) are a cosmopolitan species that are found in onion growing 2001 – Foliar Treatments 140 120 regions around the world. They have been recorded as economic pests on onion, 120 100 100 garlic, leeks, tomatoes, cucumbers and beans. 80 80 * 60 60 * Adults and nymphs feed by piercing and sucking leaf tissues, removing cell Mean # OT/Plant 40 40 * 20 OT/Plant # Mean 20 contents (Fig. 1). Light feeding results in silvery streaks while heavy feeding may 0 55 0 Days After Treatment 23 result in plant die-back. Research has shown that onion losses can exceed 40% Days After Treatment Novaluron Spinosad (L) + B. bassiani Spinosad (H)+ B. bassiani B. bassiani Novaluron Spinosad (L) Spinosad (H) when high OT populations cannot be controlled. Spinosad (H) Lambda-cyhalothrin Control Lambda-cyhalothrin Kaolin Clay Control 2002 – Foliar Treatments 25 100 20 80 15 60 40 10 * * * * Mean # OT/Plant * * 20 Mean # OT/Plant * 5 * * * 0 * 0 275 28 Days After Treatment Days After Treatment Kaolin Lambda-cyhalothrin Novaluron (L) Lambda-cyhalothrin Spinosad (L) Spinosad (H) Control Novaluron (H) Pyriproxifen Pymetrozine Control a) b) NYSAES PHOTO c) 2003 – Foliar Treatments Fig.1. -

Effects of Spinosad, Spinosad Bait, and Chloronicotinyl Insecticides on Mortality and Control of Adult and Larval Western Cherry Fruit Fly (Diptera: Tephritidae)
HORTICULTURAL ENTOMOLOGY Effects of Spinosad, Spinosad Bait, and Chloronicotinyl Insecticides on Mortality and Control of Adult and Larval Western Cherry Fruit Fly (Diptera: Tephritidae) 1 2 WEE L. YEE AND DIANE G. ALSTON J. Econ. Entomol. 99(5): 1722Ð1732 (2006) ABSTRACT Effects of spinosad, spinosad bait, and the chloronicotinyl insecticides imidacloprid and thiacloprid on mortality of the adults and larvae of western cherry fruit ßy, Rhagoletis indifferens Curran (Diptera: Tephritidae), were determined in the laboratory and the Þeld. Spinosad and spinosad bait caused higher adult mortality than imidacloprid, which caused higher mortality than thiacloprid. Only spinosad bait prevented oviposition. All materials were more toxic to adults when ingested than when topically applied. Spinosad bait had the greatest residual toxicity on leaves, killing 100% of adults when aged for 14 d in the Þeld. When materials were sprayed on infested cherries, numbers of live larvae in fruit after 8 d were lower in imidacloprid and thiacloprid than in spinosad and spinosad bait treatments, which did not differ from the control, but all materials reduced larval emergence over 30 d. In the Þeld, spinosad and spinosad bait were as effective in suppressing larval infestations as azinphos- methyl and carbaryl, whereas imidacloprid was effective in most cases and thiacloprid was generally less effective than azinphos-methyl and carbaryl. Overall, results in the laboratory and Þeld show that spinosad and chloronicotinyl insecticides differed signiÞcantly in their effectiveness against adults and larvae of R. indifferens but that spinosad, spinosad bait, and imidacloprid seem to be acceptable substitutes for organophosphate and carbamate insecticides for controlling this fruit ßy. -

Spinosad (Natroba) for Head Lice MARGARET Y
STEPS New Drug Reviews Spinosad (Natroba) for Head Lice MARGARET Y. PIO, PharmD, BCPS, CDE, Parkland Health & Hospital System, Dallas, Texas AMIMI OSAYANDE, MD, University of Texas Southwestern Medical Center, Dallas, Texas STEPS new drug reviews Spinosad (Natroba) is a prescription topical pediculicide labeled for the treatment of head cover Safety, Tolerability, lice in patients four years and older.1 In addition to its pediculicidal activity, spinosad has also Effectiveness, Price, and 2 Simplicity. Each indepen- been shown to have ovicidal activity. Unrelated to other insecticides, spinosad is produced by dent review is provided a bacterium, Saccharopolyspora spinosa.1,2 Although it is neurotoxic to lice, it does not seem to by authors who have no have a similar effect on humans or animals. financial association with the drug manufacturer. The series coordinator for Cost of full AFP is Allen F. Shaugh- Drug Dosage Dose form course* nessy, PharmD, Tufts University Family Medicine Spinosad One treatment (if warranted, a Topical suspension (0.9% strength, $147 Residency Program at (Natroba) second treatment may be used 120 mL per bottle) Cambridge Health Alli- seven days later) ance, Malden, Mass. A collection of STEPS pub- *—Estimated retail price of one treatment based on information obtained at http://www.lowestmed.com (accessed lished in AFP is available May 2, 2013). at http://www.aafp.org/ afp/steps. SAFETY TOLERABILITY Spinosad does not have any major adverse Spinosad is generally well tolerated. The effects because topical application does not most common adverse effects are erythema result in systemic exposure.1 Unlike lindane, at the site of application (3.1%), ocular spinosad has not been shown to cause neu- hyperemia (2.2%), and irritation at the site rologic toxicities, such as seizures and death. -

Managing Diseases and Insects in Home Orchards J
Managing diseases and insects in home orchards J. W. Pscheidt, H. Stoven, A. Thompson, B. Edmunds, N. Wiman, and R. Hilton In this guide, you can learn best pest management practices for your home Contents orchards. Suggested materials and times of application should have activity Table 1. Home garden/small orchard on the indicated pest. There are many fungicides and insecticides that are products ........................ 2 Importance of controlling diseases effective for managing the diseases and insects listed on the label when used and insects in commercial fruit according to the label directions. For more information, see the PNW Pest districts ......................... 3 Management Handbooks, at https://pnwhandbooks.org. Applying pesticides safely ......... 3 The best way to manage diseases and insects in your orchard is to combine Managing diseases and insects methods. Along with using pesticides, there are cultural and biological without using pesticides .......... 4 Apples .......................... 5 practices also that can help prevent or manage diseases and insects (see Pears ........................... 7 page 4). Pesticide timing and thorough spray coverage are the keys to good Peaches and Nectarines .......... 9 pest management. For good coverage, wet the leaves, twigs, and branches Apricots ........................10 thoroughly. (Note: This can be difficult with hand sprayers.) When you Cherries ........................11 use wettable powders, be sure to shake or stir the spray mix often during Prunes and Plums ...............13 application because the powders tend to settle at the bottom of the spray Walnuts ........................14 container after mixing. Hazelnuts (Filberts) .............14 To avoid excess chemical residues, be sure to use the correct rate and Moss and lichen .................15 proper interval between the last spray and harvest, as shown on the label. -

Insecticide Control of Drosophila Suzukii in Commercial Sweet Cherry Crops Under Cladding
insects Article Insecticide Control of Drosophila suzukii in Commercial Sweet Cherry Crops under Cladding Bethan Shaw 1 , Sebastian Hemer 2, Madeleine F. L. Cannon 1, Francesco Rogai 1 and Michelle T. Fountain 1,* 1 Pest and Pathology Ecology Department, NIAB EMR, New Road, East Malling, Kent ME19 6BJ, UK 2 Research and Development Department, Berry Garden Growers Ltd., Tatlingbury Oast, Five Oak Green, Tonbridge, Kent TN12 6RG, UK * Correspondence: [email protected] Received: 31 May 2019; Accepted: 2 July 2019; Published: 4 July 2019 Abstract: Drosophila suzukii Matsumura is a damaging invasive pest of sweet cherry. Using a series of laboratory leaf contact assays, semi-field, and orchard spray programs we aimed to determine the impact of insecticide programs on D. suzukii adult mortality and oviposition in cladding-protected sweet cherry crops. Tests included assessing adult D. suzukii mortality after contact with leaves sprayed either one or two weeks previously and emergence of adults from fruits. Spinosad, lambda-cyhalothrin, acetamiprid, lime, pyrethrin, deltamethrin, and cyantraniliprole all reduced fruit damage up to day 7 after application. Of these active ingredients, only spinosad, lambda-cyhalothrin, and cyantraniliprole gave satisfactory control up to 14 days. There was no significant difference in D. suzukii mortality when exposed to leaves treated either one or two weeks previously with an application of either spinosad, cyantraniliprole, or lambda-cyhalothrin; however, mortality was significantly higher than D. suzukii in contact with untreated leaves. In eight commercial orchards, fortnightly spray applications including spinosad, cyantraniliprole, and lambda-cyhalothrin gave effective control of D. suzukii until harvest with very few damaged fruits. -

Managing Pesticide Poisoning Risk and Understanding the Signs and Symptoms Clyde L
EC2505 Revised June 2018 Managing Pesticide Poisoning Risk and Understanding the Signs and Symptoms Clyde L. Ogg, Extension Educator Jan R. Hygnstrom, Project Manager Cheryl A. Alberts, Project Coordinator Erin C. Bauer, Entomology Lecturer The potential for accidents with pesticides is real. Ac- cidental exposure or overexposure to pesticides can have seri- ous consequences. While most pesticides can be used with relatively little risk when label directions are followed, some are extremely toxic and require special precautions. The Poison Control Centers receive about 90,000 calls each year related to pesticide exposures. Pesticides are re- sponsible for about 3 percent of all accidental exposures to children 5 years and younger and about 4 percent for adults. In addition, pesticides are the cause of about 3 percent of children’s deaths reported to the Poison Control Centers. Routes of Exposure Pesticides can enter the human body three ways: 1) der- mal exposure, by absorption through the skin or eyes; 2) oral exposure, through the mouth; and 3) through inhalation or respiratory exposure, by inhaling into the lungs. Some classify exposure through the eyes as ocular exposure. Dermal exposure results in absorption immediately after Figure 1. Absorption rates of different a pesticide contacts the skin or eyes. Absorption will contin- parts of the body based on the absorption ue as long as the pesticide remains in contact with the skin or of parathion into the forearm over 24 eyes. The rate at which dermal absorption occurs is different hours. for each part of the body (Figure 1). Maiback and Feldman (1974) measured the amount of the pesticide parathion absorbed by different parts of the human body over 24 hours. -

The Insecticides Act, 1968 (Act No.46 of 1968)
The Insecticides Act, 1968 (Act No.46 of 1968) An Act to regulate the import, manufactures, sale, transport, distribution and use of insecticides with a view to prevent risk to human beings or animals and for matters connected therewith. [2 nd September 1968] Be it enacted by Parliament in the Nineteenth Year of the Republic of India as follows: 1. Short title, extent and commencement. * a. This Act may be called the Insecticides Act, 1968. b. It extends to the whole of India. c. It shall come into force on such date as the Central Government may, by notification in the official Gazette, appoint and different dates may be appointed for different States and for different provisions of Act. 2. Application of other laws not barred * The provisions of this Act shall be in addition to, and not in derogation of, any other law for the time being in force. 3. Definitions- In this Act, unless the context otherwise requires- a. "animals" means animals useful to human beings and includes fish and fowl, and such kinds of wild life as the Central Government may, by notification in the official Gazette, specify, being kinds which in its opinion, it is desirable to protect or preserve; b. "Board" means the Central Insecticides Board constituted under Sec.4; c. "Central Insecticides Laboratory" means the Central Insecticides Laboratory established, or as the case may be, the institution specified under Sec.16; d. "Import" means bringing into any place within the territories to which this Act extends from a place outside those territories; e. "Insecticide" means- i. -

Ipcs Pesticide Activities
IPCS PESTICIDE ACTIVITIES The International Programme on Chemical Safety A major portion of this inventory includes sum- (IPCS) devotes significant resources to pesticides, pri- maries of toxicological evaluations of pesticides by marily for their assessment, but also for management JMPR. MRLs that have been recommended by the Joint support. The importance that IPCS places on these act- Meeting are not included. Nearly all of the MRLs have ivities is based on the widespread use of pesticides, been adopted or are under consideration by the Codex; both in agriculture and in public health, and a high level lists of Codex MRLs may be obtained from the Joint of public concern in both developed and developing FAO/WHO Food Standards Programme (see Annex 1 for countries because of the intrinsic toxicity of and potential address) or are available at the Codex web site, exposure to these chemicals. http://www.codexalimentarius.net/. This brief summary outlines the primary activities Annex 2 lists the reports and other documents of IPCS in the field of pesticides. The primary respons- prepared by JMPR. Many of the older publications that ibility for some of the activities summarized here are with are listed are out of print. The reports and evaluations programmes within WHO other than IPCS; however, that have been published by FAO may be obtained from IPCS plays a role in all of them, e.g. toxicity evaluations FAO Distribution and Sales Section, while those pub- in support of WHOPES and WHO guidelines for lished by WHO may be obtained from WHO Marketing drinking-water quality.