MF3045 Pesticide Mixtures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACEPHATE (Addendum)
3 ACEPHATE (addendum) First draft prepared by Professor P.K. Gupta 1 and Dr Angelo Moretto 2 1 Rajinder Nagar, Bareilly, UP, India; 2 Dipartimento Medicina Ambientale e Sanità Pubblica, Università di Padova, Padova, Italy Explanation..........................................................................................................3 Evaluation for acceptable daily intake.................................................................4 Biochemical aspects ......................................................................................4 Oral absorption, distribution, excretion and metabolism .......................4 Toxicological studies.....................................................................................5 Acute toxicity.........................................................................................5 Short-term studies of toxicity.................................................................6 Special studies........................................................................................7 Studies on inhibition of cholinesterase activity in vitro ..................7 Short-term study of neurotoxicity ...................................................7 Developmental neurotoxicity..........................................................9 Observations in humans ..............................................................................10 Comments..........................................................................................................12 Toxicological evaluation ...................................................................................13 -

Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects
EN58CH06-Casida ARI 5 December 2012 8:11 Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects John E. Casida1,∗ and Kathleen A. Durkin2 1Environmental Chemistry and Toxicology Laboratory, Department of Environmental Science, Policy, and Management, 2Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, California 94720; email: [email protected], [email protected] Annu. Rev. Entomol. 2013. 58:99–117 Keywords The Annual Review of Entomology is online at acetylcholinesterase, calcium channels, GABAA receptor, nicotinic ento.annualreviews.org receptor, secondary targets, sodium channel This article’s doi: 10.1146/annurev-ento-120811-153645 Abstract Copyright c 2013 by Annual Reviews. Neuroactive insecticides are the principal means of protecting crops, people, All rights reserved livestock, and pets from pest insect attack and disease transmission. Cur- ∗ Corresponding author rently, the four major nerve targets are acetylcholinesterase for organophos- phates and methylcarbamates, the nicotinic acetylcholine receptor for neonicotinoids, the γ-aminobutyric acid receptor/chloride channel for by Public Health Information Access Project on 04/29/14. For personal use only. Annu. Rev. Entomol. 2013.58:99-117. Downloaded from www.annualreviews.org polychlorocyclohexanes and fiproles, and the voltage-gated sodium channel for pyrethroids and dichlorodiphenyltrichloroethane. Species selectivity and acquired resistance are attributable in part to structural differences in binding subsites, receptor subunit interfaces, or transmembrane regions. Additional targets are sites in the sodium channel (indoxacarb and metaflumizone), the glutamate-gated chloride channel (avermectins), the octopamine receptor (amitraz metabolite), and the calcium-activated calcium channel (diamides). Secondary toxic effects in mammals from off-target serine hydrolase inhibi- tion include organophosphate-induced delayed neuropathy and disruption of the cannabinoid system. -

The Spruce Budworm, Choristoneura Fumiferana
02-01370 Spruce Budworm Bro 10/10/02 11:09 AM Page 1 MORE INFORMATION The he spruce budworm, Choristoneura fumiferana For more information on Spruce Budworms call: The Tree Line Spruce (Clemens), is the most destructive and widely (204) 945-7866. Or write: Budworm distributed forest defoliator in North America. Manitoba Conservation Forestry Branch In Manitoba T Forest Health and Ecology The destructive phase of this pest is the larval or caterpillar 200 Saulteaux Crescent Winnipeg, Manitoba R3J 3W3 stage. Massive budworm outbreaks occur periodically, Web site: www.gov.mb.ca/natres/forestry/ destroying hundreds of thousands of hectares of valuable fir and spruce. Aerial view of budworm damage In eastern Canada the budworm’s preferred food is balsam fir, Photos courtesy of Canadian Forest Service, Great Lakes Forest Research Centre, white spruce and red spruce. In Manitoba, the budworm Sault Ste. Marie, Ontario and Northern Forest Research Centre, Edmonton, Alberta. feeds primarily on white spruce and balsam fir, and, less frequently, on black spruce. 02-01370 Spruce Budworm Bro 10/10/02 11:09 AM Page 2 DESCRIPTION OF LIFE STAGES LIFE CYCLE DAMAGE CONTROL The adult moth has a wingspread of The female moth lays In light and moderate infestations Various insecticides are used 21 to 30 mm. It is grey-brown in its eggs in July on the damage is restricted to a partial against the spruce budworm to colour with silvery white patches on underside of needles. loss of new foliage, particularly in protect valuable spruce and fir the forewings. Normally, the eggs the upper crown trees. -

BOARD of PESTICIDES CONTROL January 15, 2020 Augusta Civic Center, 76 Community Drive, Kennebec/Penobscot Room, Augusta, Maine
STATE OF MAINE DEPARTMENT OF AGRICULTURE, CONSERVATION AND FORESTRY BOARD OF PESTICIDES CONTROL 28 STATE HOUSE STATION UGUSTA AINE JANET T. MILLS A , M 04333 AMANDA E. BEAL GOVERNOR COMMISSIONER BOARD OF PESTICIDES CONTROL January 15, 2020 Augusta Civic Center, 76 Community Drive, Kennebec/Penobscot Room, Augusta, Maine 1:00 - 1:30 PM Board Meeting 1:30 - 2:30 PM Public Forum On Notification 2:30 – 4:00 PM Board Meeting Continued AGENDA 1. Introductions of Board and Staff 2. Minutes of the November 8, 2019 Board Meeting Presentation By: Megan Patterson, Director Action Needed: Amend and/or Approve 3. Request for Financial Support from the Maine Mobile Health Program and the Eastern Maine Development Corporation Since 1995 the Board has supported a Migrant and Seasonal Farmworker Safety Education program. The Maine Mobile Health Program (MMHP) and Eastern Maine Development Corporation (EMDC provided training to 315 migrant agricultural workers during the 2019 season). Funding to support this effort in 2020 is being requested in the amount of $5,360, which is the same amount the Board provided in 2019. The funding has been accounted for in the Board’s FY20 budget. Presentation By: Chris Huh, Program Manager, Farmworkers Jobs Program, Eastern Maine Development Corporation Elizabeth Charles McGough, Director of Outreach, Maine Mobile Health Program MEGAN PATTERSON, DIRECTOR PHONE: (207) 287-2731 90 BLOSSOM LANE, DEERING BUILDING WWW.THINKFIRSTSPRAYLAST.ORG Action Needed: Discussion and Determination if the Board Wishes to Fund this Request 4. Request for Financial Support from the Maine State Apiarist for CLEAR Training Maine State Apiarist, Jennifer Lund, has requested funding to attend the National Certified Investigator & Inspector Basic Training held in Raleigh, North Carolina in March 2020. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

Impact of Insecticides Used to Control Spodoptera Frugiperda (J.E
RESEARCH Impact of insecticides used to control Spodoptera frugiperda (J.E. Smith) in corn on survival, sex ratio, and reproduction of Trichogramma pretiosum Riley offspring Jander R. Souza1, Geraldo A. Carvalho1, Alexandre P. Moura2*, Marcelo H.G. Couto1, and Jader B. Maia1 Corn (Zea mays L.) is cultivated in large areas and considered one of the world’s major cereal crops. There are several arthropod pests that can reduce its production such as the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lep.: Noctuidae), which is considered to be the main pest for corn. Fall armyworm is primarily controlled by insecticides. The use of biological control agents to manage this pest is growing with an emphasis on the egg parasitoid Trichogramma pretiosum Riley (Hym.: Trichogrammatidae). Thus, the aim of this research was to evaluate the impact of the following insecticides (g ai L-1) beta-cypermethrin (0.03), chlorfenapyr (0.60), chlorpyrifos (0.96), spinosad (0.16), etofenprox (0.10), triflumuron (0.08), alfa-cypermethrin/teflubenzuron (0.0425/0.0425), and lambda-cyhalothrin/thiamethoxam (0.11/0.083) on survival, sex ratio, reproduction, and T. pretiosum offspring. Distilled water was used as a control. Commercial insecticide formulations were diluted in distilled water. Bioassays used Anagasta kuehniella eggs treated with insecticides which were afterwards exposed to parasitism. Bioassays were conducted under controlled conditions at 25 ± 2 ºC, 70 ± 10% RH, and 12:12 h photoperiod. Alfa-cypermethrin/teflubenzuron, beta-cypermethrin, chlorpyrifos, chlorfenapyr, spinosad, etofenprox, and lambda-cyhalothrin/thiamethoxam reduced parasitism capacity of maternal generation females as well as the percentage of insect emergence from the F1 generation. -
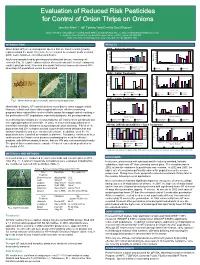
Evaluation of Reduced Risk Pesticides for Control of Onion Thrips on Onions
Evaluation of Reduced Risk Pesticides for Control of Onion Thrips on Onions Jennifer Allen1,3, Jeff Tolman2 and Cynthia Scott-Dupree3 1 – Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph ON N1G 4Y2, E-mail: [email protected] 2 – Southern Crop Protection and Food Research Centre – AAFC, London ON N5V 4T3 3 – Dept. Environmental Biology, University of Guelph, Guelph ON N1G 2W1 INTRODUCTION RESULTS Onion thrips (OT) are a cosmopolitan species that are found in onion growing 2001 – Foliar Treatments 140 120 regions around the world. They have been recorded as economic pests on onion, 120 100 100 garlic, leeks, tomatoes, cucumbers and beans. 80 80 * 60 60 * Adults and nymphs feed by piercing and sucking leaf tissues, removing cell Mean # OT/Plant 40 40 * 20 OT/Plant # Mean 20 contents (Fig. 1). Light feeding results in silvery streaks while heavy feeding may 0 55 0 Days After Treatment 23 result in plant die-back. Research has shown that onion losses can exceed 40% Days After Treatment Novaluron Spinosad (L) + B. bassiani Spinosad (H)+ B. bassiani B. bassiani Novaluron Spinosad (L) Spinosad (H) when high OT populations cannot be controlled. Spinosad (H) Lambda-cyhalothrin Control Lambda-cyhalothrin Kaolin Clay Control 2002 – Foliar Treatments 25 100 20 80 15 60 40 10 * * * * Mean # OT/Plant * * 20 Mean # OT/Plant * 5 * * * 0 * 0 275 28 Days After Treatment Days After Treatment Kaolin Lambda-cyhalothrin Novaluron (L) Lambda-cyhalothrin Spinosad (L) Spinosad (H) Control Novaluron (H) Pyriproxifen Pymetrozine Control a) b) NYSAES PHOTO c) 2003 – Foliar Treatments Fig.1. -

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

Effects of Spinosad, Spinosad Bait, and Chloronicotinyl Insecticides on Mortality and Control of Adult and Larval Western Cherry Fruit Fly (Diptera: Tephritidae)
HORTICULTURAL ENTOMOLOGY Effects of Spinosad, Spinosad Bait, and Chloronicotinyl Insecticides on Mortality and Control of Adult and Larval Western Cherry Fruit Fly (Diptera: Tephritidae) 1 2 WEE L. YEE AND DIANE G. ALSTON J. Econ. Entomol. 99(5): 1722Ð1732 (2006) ABSTRACT Effects of spinosad, spinosad bait, and the chloronicotinyl insecticides imidacloprid and thiacloprid on mortality of the adults and larvae of western cherry fruit ßy, Rhagoletis indifferens Curran (Diptera: Tephritidae), were determined in the laboratory and the Þeld. Spinosad and spinosad bait caused higher adult mortality than imidacloprid, which caused higher mortality than thiacloprid. Only spinosad bait prevented oviposition. All materials were more toxic to adults when ingested than when topically applied. Spinosad bait had the greatest residual toxicity on leaves, killing 100% of adults when aged for 14 d in the Þeld. When materials were sprayed on infested cherries, numbers of live larvae in fruit after 8 d were lower in imidacloprid and thiacloprid than in spinosad and spinosad bait treatments, which did not differ from the control, but all materials reduced larval emergence over 30 d. In the Þeld, spinosad and spinosad bait were as effective in suppressing larval infestations as azinphos- methyl and carbaryl, whereas imidacloprid was effective in most cases and thiacloprid was generally less effective than azinphos-methyl and carbaryl. Overall, results in the laboratory and Þeld show that spinosad and chloronicotinyl insecticides differed signiÞcantly in their effectiveness against adults and larvae of R. indifferens but that spinosad, spinosad bait, and imidacloprid seem to be acceptable substitutes for organophosphate and carbamate insecticides for controlling this fruit ßy. -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

Consequences of Linguistic Uncertainty for Insect Management
WATCH YOUR LANGUAGE Consequences of Linguistic Uncertainty for Insect Management THERESA M. CIRA, KATHY QUICK, AND ROBERT C. VENETTE JA'CRISPY/ISTOCK 258 AMERICAN ENTOMOLOGIST | WINTER 2019 his is a call to entomologists to consider the un- certainty introduced into our works through the language we use. Albert Einstein remarked, “Every- thing depends on the degree to which words and word-combinations correspond to the world of im- Tpression,” which makes language a “dangerous source of error and deception” (Hawking 2007). Scientists usually research and deliberately choose the language they use to propose new concepts or terminology. Often, however, terms slip into the lexicon through less systematic means. Words may seem to have meanings so obvious that broad understanding of the term may be taken for granted, but individual contexts are diverse, and to assume that a word’s meaning is unchanging across time and space is to overlook the uncertainties of lan- guage. Thus, communication through even the most common entomological vocabulary can fail. Language is a mutable, un- certain, and imperfect way of representing the world. Because language is integral to science and yet inherently imprecise, it is important for scientists to recognize and address uncertain- ty in the language of our works. Uncertainty, as defined by the Society a phenomenon is repeatedly observed and for Risk Analysis (2015), is “not knowing the individuals agree that measurements con- true value of a quantity or the future con- verge, to some degree, on a specific point, sequences of an activity,” or “imperfect or more certainty about the true nature of the incomplete information/knowledge about phenomenon can be asserted.