Volume III, Chapter 12 Caspian Tern
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Aspects of Breeding Behavior of the Royal Tern (Sterna Maxima) with Particular Emphasis on Prey Size Selectivity
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1984 Aspects of Breeding Behavior of the Royal Tern (Sterna maxima) with Particular Emphasis on Prey Size Selectivity William James Ihle College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Zoology Commons Recommended Citation Ihle, William James, "Aspects of Breeding Behavior of the Royal Tern (Sterna maxima) with Particular Emphasis on Prey Size Selectivity" (1984). Dissertations, Theses, and Masters Projects. Paper 1539625247. https://dx.doi.org/doi:10.21220/s2-4aq2-2y93 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. ASPECTS OF BREEDING BEHAVIOR OF THE ROYAL TERN (STERNA MAXIMA) n WITH PARTICULAR EMPHASIS ON PREY SIZE SELECTIVITY A Thesis Presented to The Faculty of the Department of Biology The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts by William J. Ihle 1984 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts 1MI \MLu Author Approved, April 1984 Mitchell A. Byrd m Stewart A. Ware R/ft R. Michael Erwin DEDICATION To Mom, for without her love and encouragement this thesis would not have been completed. FRONTISPIECE. Begging royal tern chick and its parent in the creche are surrounded by conspecific food parasites immediate!y after a feeding. -

Non-Living (Abiotic) Elements Shape Habitat
Forest Birds Fast Facts LIGHT: Dominated by tall trees, layered canopy = very shady, openings made from a fallen tree provide sunny areas AIR: Trees slow wind, except along forest edges and openings. Shade = cool temps WATER: Fog and rain collect in tree branches and drip to ground. SOIL: Lots of organics (needles, decaying leaves, tree trunks) makes lots of space for air and water. Soil absorbs and holds water like a sponge. Varied Thrush • Slender bill good at gleaning soft foods like insects, pillbugs, snails, worms, fruits and some seeds from ground. • Hops and pauses to look for food. Flips leaves and debris with bill. • Perching feet – three toes forward, hind toe back. • Can sing 2 separate notes at same time and breathe while singing. Common Raven Varied Thrush • Versatile beak. Eats everything from carrion and garbage, to eggs, nestlings, insects, seeds, rodents and fruit. • Strong, sturdy feet and grasping toes to manipulate food and perch. • Acrobatic flight, hops on ground. Uses sight to find food. Northern Flicker • The toes are placed two forward, two back to grip firmly, while the tail feathers are stiff and pointed to help brace while pounding. • Bill is shaped like a chisel. Flickers don’t excavate as much as other types of woodpeckers so have a slightly curved bill and less sharp. Common Raven • Flickers eat insects and are especially fond of ants (flickers will forage on the ground as well as on trees). Probe and explore crevices. • Distinctive flight – flap, dip, flap, dip • Woodpeckers have long, sticky and barbed tongues to extract bugs. -
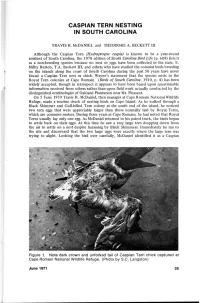
Caspian Tern Nesting in South Carolina
CASPIAN TERN NESTING IN SOUTH CAROLINA TRAVIS H. McDANIEL and THEODORE A. BECKETT III Although the Caspian Tern (Hydroprogne caspia) is known to be a year-round resident of South Carolina, the 1970 edition of South Carolina Bird Life (p. 608) lists it as a non-breeding species because no nest or eggs have been collected in the state. E. Milby Burton, T.A. Beckett III, and others who have studied the colonial birds breeding on the islands along the coast of South Carolina during the past 50 years have never found a Caspian Tern nest or chick. Wayne's statement that the species nests in the Royal Tern colonies at Cape Romain (Birds of South Carolina, 1910, p. 4) has been widely accepted, though in retrospect it appears to have been based upon questionable information received from others rather than upon field work actually conducted by the distinguished ornithologist of Oakland Plantation near Mt. Pleasant. On 5 June 1970 Travis H. McDaniel, then manager at Cape Romain National Wildlife Refuge, made a routine check of nesting birds on Cape Island. As he walked through a Black Skimmer and Gull-billed Tern colony at the south end of the island, he noticed two tern eggs that were appreciably larger than those normally laid by Royal Terns, which are common nesters. During three years at Cape Romain, he had noted that Royal Terns usually lay only one egg. As McDaniel returned to his patrol truck, the birds began to settle back on their eggs. At this time he saw a very large tern dropping down from the air to settle on a nest despite harassing by Black Skimmers. -

Breeding Birds of the Texas Coast
Roseate Spoonbill • L 32”• Uncom- Why Birds are Important of the mon, declining • Unmistakable pale Breeding Birds Texas Coast pink wading bird with a long bill end- • Bird abundance is an important indicator of the ing in flat “spoon”• Nests on islands health of coastal ecosystems in vegetation • Wades slowly through American White Pelican • L 62” Reddish Egret • L 30”• Threatened in water, sweeping touch-sensitive bill •Common, increasing • Large, white • Revenue generated by hunting, photography, and Texas, decreasing • Dark morph has slate- side to side in search of prey birdwatching helps support the coastal economy in bird with black flight feathers and gray body with reddish breast, neck, and Chuck Tague bright yellow bill and pouch • Nests Texas head; white morph completely white – both in groups on islands with sparse have pink bill with Black-bellied Whistling-Duck vegetation • Preys on small fish in black tip; shaggy- • L 21”• Lo- groups looking plumage cally common, increasing • Goose-like duck Threats to Island-Nesting Bay Birds Chuck Tague with long neck and pink legs, pinkish-red bill, Greg Lavaty • Nests in mixed- species colonies in low vegetation or on black belly, and white eye-ring • Nests in tree • Habitat loss from erosion and wetland degradation cavities • Occasionally nests in mesquite and Brown Pelican • L 51”• Endangered in ground • Uses quick, erratic movements to • Predators such as raccoons, feral hogs, and stir up prey Chuck Tague other woody vegetation on bay islands Texas, but common and increasing • Large -

Predator and Competitor Management Plan for Monomoy National Wildlife Refuge
Appendix J /USFWS Malcolm Grant 2011 Fencing exclosure to protect shorebirds from predators Predator and Competitor Management Plan for Monomoy National Wildlife Refuge Background and Introduction Background and Introduction Throughout North America, the presence of a single mammalian predator (e.g., coyote, skunk, and raccoon) or avian predator (e.g., great horned owl, black-crowned night-heron) at a nesting site can result in adult bird mortality, decrease or prevent reproductive success of nesting birds, or cause birds to abandon a nesting site entirely (Butchko and Small 1992, Kress and Hall 2004, Hall and Kress 2008, Nisbet and Welton 1984, USDA 2011). Depredation events and competition with other species for nesting space in one year can also limit the distribution and abundance of breeding birds in following years (USDA 2011, Nisbet 1975). Predator and competitor management on Monomoy refuge is essential to promoting and protecting rare and endangered beach nesting birds at this site, and has been incorporated into annual management plans for several decades. In 2000, the Service extended the Monomoy National Wildlife Refuge Nesting Season Operating Procedure, Monitoring Protocols, and Competitor/Predator Management Plan, 1998-2000, which was expiring, with the intent to revise and update the plan as part of the CCP process. This appendix fulfills that intent. As presented in chapter 3, all proposed alternatives include an active and adaptive predator and competitor management program, but our preferred alternative is most inclusive and will provide the greatest level of protection and benefit for all species of conservation concern. The option to discontinue the management program was considered but eliminated due to the affirmative responsibility the Service has to protect federally listed threatened and endangered species and migratory birds. -

Dwergstern3.Pdf
99 PRIMARY MOULT, BODY MASS AND MOULT MIGRATION OF LITTLE TERN STERNAALBIFRONS IN NE ITALY GIUSEPPE CHERUBINI, LORENZO SERRA & NICOLA BACCETTI Cherubini G., L. Serra & N. Baccetti 1996. Primary moult, body mass and moult migration of Little Tern Sterna albifrons in NE Italy. Ardea 84: 99 114. Large post-breeding gatherings of Little Terns Sterna albifrons are regu larly observed in the Lagoon of Venice, Italy. Here, during five consecutive \ 1/ trapping seasons, 2956 birds were examined and ringed. Their breeding area, as indicated by 163 direct recoveries (mainly juveniles, ringed as chicks), spans over a broad sector of the Adriatic coasts, with colonies lo cated up to 133 km far. During their stay at the study area, adults undergo an almost complete moult. Two partial primary moult cycles can be ob \ served, the first of them being suspended when 2-4 outermost long primar ies have not yet been shed. Pre-migratory body mass build-up, enough for a ~/ flight longer than 1000 km, takes place during the very last days before de parture to the winter quarters, in most cases when the moult has reached a I suspended stage. Active primary moult and body mass increase do overlap in late moulting birds (after 27 August), indicating that the two processes are compatible, in case of time shortage. Post-breeding movements to the Lagoon of Venice seem to fit most requisites of moult migration. Key words: Sterna albifrons - Italy - biometrics - moult migration - ringing - fattening Istituto Nazionale per la Fauna Selvatica, via Ca' Fornacetta 9,1-40064 Oz zano Emilia BO, Italy. -

Lowrie, K., M. Friesen, D. Lowrie, and N. Collier. 2009. Year 1 Results Of
2009 Year 1 Results of Seabird Breeding Atlas of the Lesser Antilles Katharine Lowrie, Project Manager Megan Friesen, Research Assistant David Lowrie, Captain and Surveyor Natalia Collier, President Environmental Protection In the Caribbean 200 Dr. M.L. King Jr. Blvd. Riviera Beach, FL 33404 www.epicislands.org Contents INTRODUCTION ................................................................................................................................... 3 GENERAL METHODS ............................................................................................................................ 4 Field Work Overview ........................................................................................................................... 4 Water‐based Surveys ...................................................................................................................... 4 Data Recorded ................................................................................................................................. 5 Land‐based Surveys ......................................................................................................................... 5 Large Colonies ................................................................................................................................. 6 Audubon’s Shearwater .................................................................................................................... 7 Threats Survey Method ................................................................................................................. -

Nest Spacing in Elegant Terns: Hexagonal Packing Revisited Charles T
WESTERN BIRDS Volume 39, Number 2, 2008 NEST SPACING IN ELEGANT TERNS: HEXAGONAL PACKING REVISITED CHARLES T. COLLINS and MICHAEL D. TAYLOR, Department of Biological Sci- ences, California State University, Long Beach, California 90840 (current address of Taylor: Santiago Canyon College, 8045 East Chapman Ave., Orange, California 92869); [email protected] ABSTRACT: Within an important breeding colony in southern California, Elegant Terns (Thalasseus elegans) nest in one to several tightly packed clusters. Inter-nest distances within these clusters average 31.2 cm. This value is less than that reported for the larger-bodied Royal Tern (T. maximus) and Great Crested Tern (T. bergii). For Elegant Terns, the modal number of adjacent nests was six (range 5–7). This type of nest arrangement has been previously described as hexagonal packing and now appears to be typical of all Thalasseus terns for which data are available. Many seabirds nest in large, often traditional, colonies (Coulson 2002, Schreiber and Burger 2002). The ontogeny of annual colony formation has been reviewed by Kharitonov and Siegel-Causey (1988), and the evolution- ary processes which have led to coloniality have been considered by a number of authors (Lack 1968, Fischer and Lockley 1974, Wittenburger and Hunt 1985, Siegel-Causey and Kharitonov 1990, Coulson 2002). Seabird colonies may be rather loosely organized aggregations of breeding pairs of one to several species at a single site. At the other extreme, they may be dense, tightly packed, largely monospecific clusters where distances between nests are minimal. A graphic example of the latter is the dense clustering of nests recorded for several species of crested terns (Buckley and Buckley 1972, 2002, Hulsman 1977, Veen 1977, Symens and Evans 1993, Burness et al. -

Roseate Tern Sterna Dougallii
COSEWIC Assessment and Update Status Report on the Roseate Tern Sterna dougallii in Canada Roseate Tern. Diane Pierce © 1995 ENDANGERED 2009 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2009. COSEWIC assessment and update status report on the Roseate Tern Sterna dougallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 48 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous reports: COSEWIC. 1999. COSEWIC assessment and update status report on the Roseate Tern Sterna dougallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 28 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Whittam, R.M. 1999. Update COSEWIC status report on the Roseate Tern Sterna dougallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-28 pp. Kirkham, I.R. and D.N. Nettleship. 1986. COSEWIC status report on the Roseate Tern Sterna dougallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 49 pp. Production note: COSEWIC would like to acknowledge Becky Whittam for writing the status report on the Roseate Tern Sterna dougallii in Canada, prepared under contract with Environment Canada, overseen and edited by Richard Cannings and Jon McCracken, Co-chairs, COSEWIC Birds Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Sterne de Dougall (Sterna dougallii) au Canada – Mise à jour. -

Common Tern Sterna Hirundo
Common Tern Sterna hirundo Dick Young CURRENT STATUS: In Pennsylvania, the common tern is endangered and protected under the Game and Wildlife Code. In the northeastern United States, the U.S. Fish and Wildlife Service lists the common tern as a migratory bird of conservation concern in the lower Great Lakes region. Nationally, they are not listed as an endangered/threatened species. All migratory birds are protected under the federal Migratory Bird Treaty Act of 1918. POPULATION TREND: Common terns (Sterna hirundo) have not nested successfully in Pennsyl- vania for many years; however the potential for nesting still exists. The only location with suitable nest- ing habitat is their historic nest site at Presque Isle State Park in Erie County. They once nested in abun- dance on Gull Point at the east end of the park, where more than 100 pairs were recorded in the early 1930s. Frequent disturbances by recreational beach-goers led to abandonment of the site. In 1985, the common tern was determined to be “extirpated,” or absent as a nesting species, in the Pennsylvania Bio- logical Survey’s Species of Special Concern in Pennsylvania. In 1999 the species was upgraded to “endangered” after a nesting pair was found in the newly protected Gull Point Natural Area at Presque Isle. The vulnerable eggs were destroyed before hatching, however. More recently, there were unsuc- cessful nesting attempts in 2012 and 2014. Despite these disappointments, common terns are fairly common to abundant migrants along Lake Erie, offering hope that breeding birds will stay to nest once again in Pennsylvania. -

Site Fidelity and Colony Dynaimcs of Caspian Terns Nesting at East
AN ABSTRACT OF THE THESIS OF Stefanie Collar for the degree of Master of Science in Wildlife Science presented on December 12, 2013. Title: Site Fidelity and Colony Dynamics of Caspian Terns Nesting at East Sand Island, Columbia River Estuary, Oregon, USA. Abstract approved: ____________________________________________________________________ Daniel D. Roby Fidelity to breeding sites in colonial birds is an adaptive trait thought to have evolved to enhance reproductive success by reducing search time for breeding habitat, allowing earlier nest initiation, facilitating mate retention, and reducing uncertainty of predator presence and food availability. Studying a seabird that has evolved relatively low colony fidelity, such as the Caspian tern, allowed me to explore the influence of stable nesting habitat on fidelity and nest site selection. The Caspian tern (Hydroprogne caspia) breeding colony on East Sand Island (ESI) in the Columbia River estuary is the largest colony of its kind in the world. This colony has experienced a decade of declining nesting success, culminating with the failure of the colony to produce any young in 2011. The objective of my study was to understand the dynamics of this Caspian tern super-colony by investigating the actions of breeding individuals over two seasons, as well as the behavior of the colony as a whole from 2001-2011. I was interested in (1) the degree of nest site fidelity exhibited by breeding terns in successive years and its relationship to reproductive success, and (2) how the interaction of top-down and bottom-up forces influenced average nesting success across the entire colony, and caused the observed trends in nesting success at the East Sand Island colony from 2001 to 2011. -

Pacific Seabird Program Business Plan (Dawson Et Al
National Fish and Wildlife Foundation Business Plan for Pacific Seabirds (Update) September 2016 Pacific Seabirds | 1 Purpose of a Business Plan The purpose of a NFWF business plan is to provide a detailed blueprint of the strategies and resources required to achieve the desired conservation outcomes. The strategies discussed in this plan do not represent solely the Foundation’s view of the actions necessary to achieve the identified conservation goals, but instead reflect the majority view of the many federal, state, academic, and organizational experts that were consulted during plan development. This plan is not meant to duplicate ongoing work but rather to invest in areas where gaps might exist so as to support the efforts of the larger conservation community. Acknowledgements We thank everyone who contributed to this business plan. We are especially grateful to the seabird experts, funding partners, and working group teams who took the time to develop, contribute, and review material. We acknowledge the contributions of Dantzker Consulting, Advanced Conservation Strategies, and Clarus Research for their evaluation of the Pacific Seabird Program and recommendations for continued implementation of this program. We also wish to acknowledge the valuable input resulting from discussions and written material provided by implementation and funding partners including (but not limited to): The American Bird Conservancy, BirdLife International, The David and Lucille Packard Foundation, The Farallon Institute, Island Conservation, the National Audubon Society, National Park Service, National Oceanographic and Atmospheric Administration, Oikonos, University of California Santa Cruz Coastal Conservation Action Lab, The University of Washington, The U.S. Fish and Wildlife Service, The U.S.