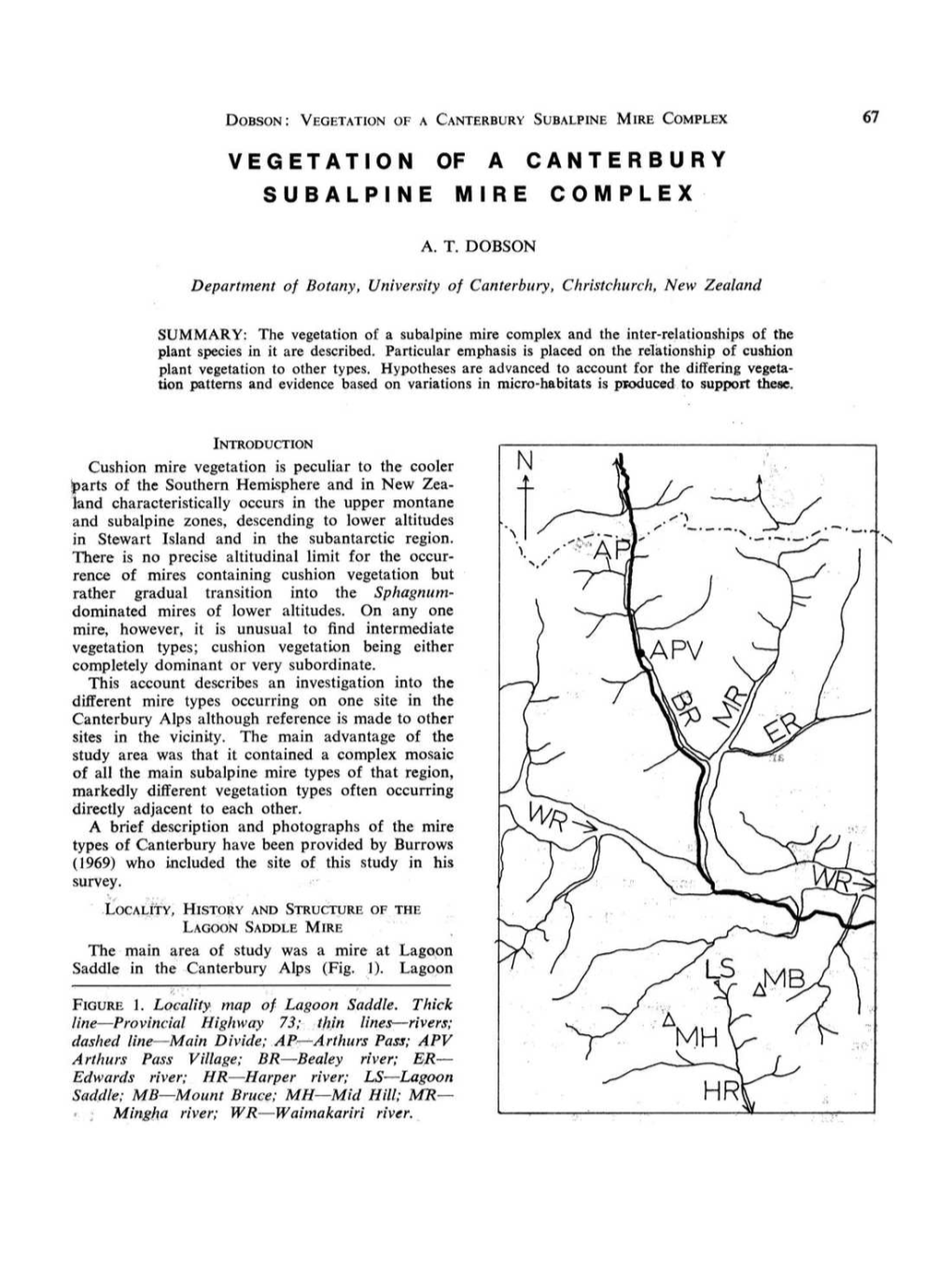
Subalpine Mire Complex 67
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VESSELS in ERIOCAULACEAE By
IAWA Journ al, Vol. 17 (2), 1996: 183-204 VESSELS IN ERIOCAULACEAE by Jennifer A. Thorsch & Vernon I. Cheadle I Department of Ecology, Evolution and Marine Biology, University of California, Santa Barbara, CA 93 106, U.S.A. SUMMARY The occ urrence and level of specialization of vesse ls in 70 species representing 12 genera of Eriocaulaceae are presented. In alI species of Eriocaulaceae and in alI parts of the plant examined, vessels with simple perforations have been identified . Correlations between level of specia lization of vessel members and ecological conditi ons are reported for species from diverse habitats and species with distinct differences in habit. The pattern of origin and specialization of tracheary celIs in Erio caulaceae was compared to tracheary data for Xyridaceae , Rapateaceae, Restionaceae and Centrolepidaceae. The evolutionary position of these families has been regarded as close to Eriocaulaceae. Key words: Eriocaulaceae, vessels, perforation plates, phylogenetic posi tion. INTRODUCTION This paper provides detailed information about perforation plates of vessels in Erio caulaceae, the 29th family we have similarly examined in monocotyledons. The near ly complete list of families analyzed in detail is given in the literature cited in the paper on Commelinales (Cheadle & Kosakai 1980). Our studies on the tracheary elements in monocotyledons have included families and species from a broad range of habits, habitats and geographical sites around the world. Families for study were selected based on three criteria: I) presence of alI plant parts, 2) variety of habits and habitats, and 3) broad representation of the species within the family. The data on tracheids and vessels from these broadly based studies led to the folIowing brief conclusions. -

Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 3 2006 Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University Thomas Janssen Muséum National d'Histoire Naturelle Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Bremer, Kåre and Janssen, Thomas (2006) "Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 3. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/3 Aliso 22, pp. 22-27 © 2006, Rancho Santa Ana Botanic Garden GONDWANAN ORIGIN OF MAJOR MONO COT GROUPS INFERRED FROM DISPERSAL-VICARIANCE ANALYSIS KARE BREMERl.3 AND THOMAS JANSSEN2 lDepartment of Systematic Botany, Evolutionary Biology Centre, Norbyvagen l8D, SE-752 36 Uppsala, Sweden; 2Museum National d'Histoire Naturelle, Departement de Systematique et Evolution, USM 0602: Taxonomie et collections, 16 rue Buffon, 75005 Paris, France 3Corresponding author ([email protected]) ABSTRACT Historical biogeography of major monocot groups was investigated by biogeographical analysis of a dated phylogeny including 79 of the 81 monocot families using the Angiosperm Phylogeny Group II (APG II) classification. Five major areas were used to describe the family distributions: Eurasia, North America, South America, Africa including Madagascar, and Australasia including New Guinea, New Caledonia, and New Zealand. In order to investigate the possible correspondence with continental breakup, the tree with its terminal distributions was fitted to the geological area cladogram «Eurasia, North America), (Africa, (South America, Australasia») and to alternative area cladograms using the TreeFitter program. -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

Vicariance, Climate Change, Anatomy and Phylogeny of Restionaceae
Botanical Journal of the Linnean Society (2000), 134: 159–177. With 12 figures doi:10.1006/bojl.2000.0368, available online at http://www.idealibrary.com on Under the microscope: plant anatomy and systematics. Edited by P. J. Rudall and P. Gasson Vicariance, climate change, anatomy and phylogeny of Restionaceae H. P. LINDER FLS Bolus Herbarium, University of Cape Town, Rondebosch 7701, South Africa Cutler suggested almost 30 years ago that there was convergent evolution between African and Australian Restionaceae in the distinctive culm anatomical features of Restionaceae. This was based on his interpretation of the homologies of the anatomical features, and these are here tested against a ‘supertree’ phylogeny, based on three separate phylogenies. The first is based on morphology and includes all genera; the other two are based on molecular sequences from the chloroplast genome; one covers the African genera, and the other the Australian genera. This analysis corroborates Cutler’s interpretation of convergent evolution between African and Australian Restionaceae. However, it indicates that for the Australian genera, the evolutionary pathway of the culm anatomy is much more complex than originally thought. In the most likely scenario, the ancestral Restionaceae have protective cells derived from the chlorenchyma. These persist in African Restionaceae, but are soon lost in Australian Restionaceae. Pillar cells and sclerenchyma ribs evolve early in the diversification of Australian Restionaceae, but are secondarily lost numerous times. In some of the reduction cases, the result is a very simple culm anatomy, which Cutler had interpreted as a primitively simple culm type, while in other cases it appears as if the functions of the ribs and pillars may have been taken over by a new structure, protective cells developed from epidermal, rather than chlorenchyma, cells. -

An Annotated Checklist of Tasmanian Mosses
15 AN ANNOTATED CHECKLIST OF TASMANIAN MOSSES by P.I Dalton, R.D. Seppelt and A.M. Buchanan An annotated checklist of the Tasmanian mosses is presented to clarify the occurrence of taxa within the state. Some recently collected species, for which there are no published records, have been included. Doubtful records and excluded speciei. are listed separately. The Tasmanian moss flora as recognised here includes 361 species. Key Words: mosses, Tasmania. In BANKS, M.R. et al. (Eds), 1991 (3l:iii): ASPECTS OF TASMANIAN BOTANY -- A TR1BUn TO WINIFRED CURTIS. Roy. Soc. Tasm. Hobart: 15-32. INTRODUCTION in recent years previously unrecorded species have been found as well as several new taxa described. Tasmanian mosses received considerable attention We have assigned genera to families followi ng Crosby during the early botanical exploration of the antipodes. & Magill (1981 ), except where otherwise indicated in One of the earliest accounts was given by Wilson (1859), the case of more recent publications. The arrangement who provided a series of descriptions of the then-known of families, genera and species is in alphabetic order for species, accompanied by coloured illustrations, as ease of access. Taxa known to occur in Taslnania ami Part III of J.D. Hooker's Botany of the Antarctic its neighbouring islands only are listed; those for Voyage. Although there have been a number of papers subantarctic Macquarie Island (politically part of since that time, two significant compilations were Tasmania) are not treated and have been presented published about the tum of the century. The first was by elsewhere (Seppelt 1981). -

(Centrolepidaceae) in Australia
J. Adelaide Bot. Gard. 15(1): 1-63 (1992) A TAXONOMIC REVISION OF CENTROLEPIS (CENTROLEPIDACEAE) IN AUSTRALIA D. A. Cooke Animal and Plant Control Commission of South Australia GPO Box 1671, Adelaide, South Australia 5001 Abstract Centrdepis in Australia is revised and twenty species are recognised. This revision is based on morphological features that are discussed in relation to the biology of the genus. One new species, C. curta, and a new subspecies, C. strigosa subsp. rupestris, are described and illustrated. The new combinations C. monogyna subsp. paludicola and C. strigosa subsp. pulvinata are made. Introduction Centrolepis is a genus of small annual and perennial monocots. It forms, with Aphelia and Gaimardia, the minor family Centrolepidaceae. The family has its main centre of diversity in Australia with 29 species; a few occur in New Zealand, south-eastern Asia and South America. The close affinity of the Centrolepidaceae to the Restionaceae, and its remoteness from the two genera segregated by Hamann (1976) as the Hydatellaceae, are widely recognised in contemporary systems of classification (Cronquist, 1981; Dahlgren & Clifford, 1982; Takhtajan, 1980). Taxonomic history The genus first became known from material of the near-coastal species sent to Europe by the early botanist-explorers and collectors. In 1770 Banks and Solander on the Endeavour collected specimens of Centrolepis, now referred to C. banksii and C. exserta, that they tentatively labelled as species of Schoenus (Cyperaceae). Labillardière (1804) based the new genus Centrolepis, which he placed under Monandria Monogynia in the Linnaean system, on a Tasmanian specimen. Robert Brown (1810), using Banks' and Solander's material and his own collections from the voyage of the Investigator around Australia in 1801-4, drafted manuscript epithets for a further twelve Centrolepis species. -

Centrolepidosporium Sclerodermum, Gen. Et Sp. Nov. (Ustilaginomycetes) from Australia
MYCOLOGIA BALCANICA 4: 1–4 (2007) 1 Centrolepidosporium sclerodermum, gen. et sp. nov. (Ustilaginomycetes) from Australia Roger G. Shivas * & Kálmán Vánky Plant Pathology Herbarium, Queensland Department of Primary Industries and Fisheries, 80 Meiers Road, Indooroopilly, Queensland 4068, Australia Herbarium Ustilaginales Vánky (H.U.V.), Gabriel-Biel-Str. 5, D-72076 Tübingen, Germany Received 24 November 2006 / Accepted: 14 February 2007 Abstract. A new genus, Centrolepidosporium, is proposed to accommodate a new smut fungus, C. sclerodermum, collected in Australia on Centrolepis exserta. Th e new species is unique in that it produces tightly packed spores in spore balls surrounded by a cortex of sterile cells. Th is is the fi rst report of a smut fungus on the plant family Centrolepidaceae. Key words: new species, smut fungi, Sporisorium, Tilletia, Tranzscheliella, Urocystis, Ustilaginomycetes Introduction the Northern Territory, Australia, on Centrolepis exserta (R. Br.) Roem. & Schult., which grows across northern Australia Th e Centrolepidaceae is a family of 3 genera (Aphelia, on the margins of streams and wetlands, and moist sites in Centrolepis and Gaimardia) and about 36 species (Mabberley woodland or grassland, mainly on sandy alluvial soils (Cooke 1998), mostly occurring in Australia but also found in New 1992). Th is collection represents both a new species and also Zealand, throughout south-east Asia to Laos with an outlying a new genus (comp. Vánky 2002). Gaimardia species in South America (Cooke 1988). It is a family of small, sedge-like annuals and perennials with greatly reduced unisexual fl owers combined into a pseudanthium, Materials and Methods which is a highly condensed unit infl orescence analogous to a bisexual fl ower but composed of 2-many reduced unisexual Sorus and spore characteristics were studied using dried fl owers (Cooke 1988). -

Flora of New Zealand Seed Plants
FLORA OF NEW ZEALAND SEED PLANTS CENTROLEPIDACEAE K.A. FORD Fascicle 2 – JUNE 2014 © Landcare Research New Zealand Limited 2014. This copyright work is licensed under the Creative Commons Attribution 3.0 New Zealand license. Attribution if redistributing to the public without adaptation: “Source: Landcare Research” Attribution if making an adaptation or derivative work: “Sourced from Landcare Research” CATALOGUING IN PUBLICATION Ford, Kerry A. (Kerry Alison) Flora of New Zealand [electronic resource] : seed plants. Fascicle 2, Centrolepidaceae / K.A. Ford. -- Lincoln, N.Z. : Manaaki Whenua Press, 2014. 1 online resource ISBN 978-0-478-34764-7 (pdf) ISBN 978-0-478-34762-3 (set) 1.Phanerogams -- New Zealand - Identification. I. Title. II. Manaaki Whenua-Landcare Research New Zealand Ltd. DOI: 10.7931/J2H41PBX This work should be cited as: Ford, K.A. 2014: Centrolepidaceae. In: Breitwieser, I.; Brownsey, P.J.; Heenan, P.B.; Wilton, A.D. Flora of New Zealand - Seed Plants. Fascicle 2. Manaaki Whenua Press, Lincoln. http://dx.doi.org/10.7931/J2H41PBX Cover image: Centrolepis ciliata, habit of cushion (near Lake Te Anau). Contents Introduction..............................................................................................................................................1 Taxa Centrolepidaceae Endl. ..................................................................................................................... 2 Centrolepis Labill. ............................................................................................................................. -

Assembling the Tree of the Monocotyledons: Plastome Sequence Phylogeny and Evolution of Poales Author(S) :Thomas J
Assembling the Tree of the Monocotyledons: Plastome Sequence Phylogeny and Evolution of Poales Author(s) :Thomas J. Givnish, Mercedes Ames, Joel R. McNeal, Michael R. McKain, P. Roxanne Steele, Claude W. dePamphilis, Sean W. Graham, J. Chris Pires, Dennis W. Stevenson, Wendy B. Zomlefer, Barbara G. Briggs, Melvin R. Duvall, Michael J. Moore, J. Michael Heaney, Douglas E. Soltis, Pamela S. Soltis, Kevin Thiele, and James H. Leebens-Mack Source: Annals of the Missouri Botanical Garden, 97(4):584-616. 2010. Published By: Missouri Botanical Garden DOI: URL: http://www.bioone.org/doi/full/10.3417/2010023 BioOne (www.bioone.org) is a a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/ page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non- commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. ASSEMBLING THE TREE OF THE Thomas J. Givnish,2 Mercedes Ames,2 Joel R. MONOCOTYLEDONS: PLASTOME McNeal,3 Michael R. McKain,3 P. Roxanne Steele,4 Claude W. dePamphilis,5 Sean W. -

Vale of Belvoir Reserve Supplement Contents Key
BUSH BLITZ SPECIES DISCOVERY PrOGRAM Vale of Belvoir Reserve Supplement Contents Key Appendix A: Species Lists 3 ¤ = Previously recorded on the reserve and Fauna 4 found on this survey Vertebrates 4 * = New record for this reserve Birds 4 ^ = Exotic/Pest Fishes 5 # = EPBC listed Frogs 5 ~ = TSP listed Putative new species Mammals 6 Previously recorded on the reserve but not found on Reptiles 6 this survey Invertebrates 7 Bees 7 EPBC = Environment Protection and Biodiversity Butterflies and Moths 7 Conservation Act 1999 (Commonwealth) Flies 7 TSP = Threatened Species Protection Act 1995 Beetles 7 (Tasmania) True Bugs 8 Grasshoppers 8 Dragonflies 8 Caddisflies 9 Millipedes 9 Spiders 10 Crustaceans 10 Snails and Slugs 11 Flora 12 Flowering Plants 12 Gymnosperms 13 Ferns and Fern Allies 13 Liverworts 14 Mosses 14 Fungi 15 Appendix B: Rare and Threatened Species 17 Appendix C: Exotic and Pest Species 19 2 Bush Blitz survey report — Tasmania 2010 Appendix A: Species Lists Nomenclature and taxonomy used in this appendix are consistent with that from the Australian Faunal Directory (AFD), the Australian Plant Name Index (APNI) and the Australian Plant Census (APC). Current at April 2012 Vale of Belvoir Reserve Supplement 3 Fauna Vertebrates Birds Family Species Common name Acanthizidae Acanthiza ewingii Tasmanian Thornbill Acanthiza pusilla * Brown Thornbill Acanthornis magna Scrubtit Calamanthus fuliginosus * Striated Fieldwren Sericornis frontalis * White-browed Scrubwren Sericornis humilis * Tasmanian Scrubwren Accipitridae Aquila audax * -

Nuclear Genes, Matk and the Phylogeny of the Poales
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2018 Nuclear genes, matK and the phylogeny of the Poales Hochbach, Anne ; Linder, H Peter ; Röser, Martin Abstract: Phylogenetic relationships within the monocot order Poales have been well studied, but sev- eral unrelated questions remain. These include the relationships among the basal families in the order, family delimitations within the restiid clade, and the search for nuclear single-copy gene loci to test the relationships based on chloroplast loci. To this end two nuclear loci (PhyB, Topo6) were explored both at the ordinal level, and within the Bromeliaceae and the restiid clade. First, a plastid reference tree was inferred based on matK, using 140 taxa covering all APG IV families of Poales, and analyzed using parsimony, maximum likelihood and Bayesian methods. The trees inferred from matK closely approach the published phylogeny based on whole-plastome sequencing. Of the two nuclear loci, Topo6 supported a congruent, but much less resolved phylogeny. By contrast, PhyB indicated different phylo- genetic relationships, with, inter alia, Mayacaceae and Typhaceae sister to Poaceae, and Flagellariaceae in a basally branching position within the Poales. Within the restiid clade the differences between the three markers appear less serious. The Anarthria clade is first diverging in all analyses, followed by Restionoideae, Sporadanthoideae, Centrolepidoideae and Leptocarpoideae in the matK and Topo6 data, but in the PhyB data Centrolepidoideae diverges next, followed by a paraphyletic Restionoideae with a clade consisting of the monophyletic Sporadanthoideae and Leptocarpoideae nested within them. The Bromeliaceae phylogeny obtained from Topo6 is insufficiently sampled to make reliable statements, but indicates a good starting point for further investigations. -

Co-Extinction of Mutualistic Species – an Analysis of Ornithophilous Angiosperms in New Zealand
DEPARTMENT OF BIOLOGICAL AND ENVIRONMENTAL SCIENCES CO-EXTINCTION OF MUTUALISTIC SPECIES An analysis of ornithophilous angiosperms in New Zealand Sandra Palmqvist Degree project for Master of Science (120 hec) with a major in Environmental Science ES2500 Examination Course in Environmental Science, 30 hec Second cycle Semester/year: Spring 2021 Supervisor: Søren Faurby - Department of Biological & Environmental Sciences Examiner: Johan Uddling - Department of Biological & Environmental Sciences “Tui. Adult feeding on flax nectar, showing pollen rubbing onto forehead. Dunedin, December 2008. Image © Craig McKenzie by Craig McKenzie.” http://nzbirdsonline.org.nz/sites/all/files/1200543Tui2.jpg Table of Contents Abstract: Co-extinction of mutualistic species – An analysis of ornithophilous angiosperms in New Zealand ..................................................................................................... 1 Populärvetenskaplig sammanfattning: Samutrotning av mutualistiska arter – En analys av fågelpollinerade angiospermer i New Zealand ................................................................... 3 1. Introduction ............................................................................................................................... 5 2. Material and methods ............................................................................................................... 7 2.1 List of plant species, flower colours and conservation status ....................................... 7 2.1.1 Flower Colours .............................................................................................................