Control of Ameloblast Differentiation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
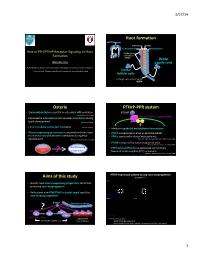
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes
Cells and Materials Volume 3 Number 2 Article 8 1993 Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes H. Lesot Institut de Biologie Médicale C. Begue-Kirn Institut de Biologie Médicale M. D. Kubler Institut de Biologie Médicale J. M. Meyer Institut de Biologie Médicale A. J. Smith Dental School, Birmingham See next page for additional authors Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Lesot, H.; Begue-Kirn, C.; Kubler, M. D.; Meyer, J. M.; Smith, A. J.; Cassidy, N.; and Ruch, J. V. (1993) "Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes," Cells and Materials: Vol. 3 : No. 2 , Article 8. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes Authors H. Lesot, C. Begue-Kirn, M. D. Kubler, J. M. Meyer, A. J. Smith, N. Cassidy, and J. V. Ruch This article is available in Cells and Materials: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 Cells and Materials, Vol. 3, No. 2, 1993 (Pages201-217) 1051-6794/93$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA EXPERIMENTAL INDUCTION OF ODONTOBLAST DIFFERENTIATION AND STIMULATION DURING REPARATIVE PROCESSES 1 1 1 2 2 1 H. -

Human Identification by Amelogenin Test in Libyans
American Journal of www.biomedgrid.com Biomedical Science & Research ISSN: 2642-1747 --------------------------------------------------------------------------------------------------------------------------------- Research Article Copyright@ Samir Elmrghni Human Identification by Amelogenin Test in Libyans Samir Elmrghni* and Mahmoud Kaddura Department of Forensic Medicine and Toxicology, University of Benghazi, Libya *Corresponding author: Samir Elmrghni, Faculty of Medicine, Department of Forensic Medicine and Toxicology, University of Benghazi-Libya, Benghazi, Libya. To Cite This Article: Samir Elmrghni. Human Identification by Amelogenin Test in Libyans. Am J Biomed Sci & Res. 2019 - 3(6). AJBSR. MS.ID.000737. DOI: 10.34297/AJBSR.2019.03.000737 Received: May 25, 2019 | Published: July 11, 2019 Abstract Sex typing is essential in medical diagnosis of sex-linked disease and forensic science. Gender for criminal evidence of offender is usually as reported the anomalous amelogenin results of 2 male samples (out of 238 males) represented as females (Y deletions) and another 2 samples the initial information for investigation. For individualization, identification of gender is performed in addition to the STR markers recently. We amelogenin results of the controversial samples, DNA was further used in SRY and Y-STR typing. All samples typed as males but two showed with with (X deletions) in Benghazi (Libya). The frequency in both was about 0.8%. Higher than those of the other populations reported. To confirm X chromosomes. From the results, it was highly suggested that for the controversial cases of human gender identification with amelogenin tests, amplificationKeywords: Amelogenin; of SRY gene Libyans or/and Y-STR markers will be adopted to confirm the gender [1]. Introduction gene (AMG) was precisely mapped in the p22 region on the X systems used to determine if the sample being tested is of male gene was first isolated and sequenced [2]. -

Mutational Analysis of Candidate Genes in 24 Amelogenesis
Eur J Oral Sci 2006; 114 (Suppl. 1): 3–12 Copyright Ó Eur J Oral Sci 2006 Printed in Singapore. All rights reserved European Journal of Oral Sciences Jung-Wook Kim1,2, James P. Mutational analysis of candidate genes Simmer1, Brent P.-L. Lin3, Figen Seymen4, John D. Bartlett5, Jan C.-C. in 24 amelogenesis imperfecta families Hu1 1University of Michigan School of Dentistry, University of Michigan Dental Research 2 Kim J-W, Simmer JP, Lin BP-L, Seymen F, Bartlett JD, Hu JC-C. Mutational analysis Laboratory, Ann Arbor, MI, USA; Seoul National University, College of Dentistry, of candidate genes in 24 amelogenesis imperfecta families. Eur J Oral Sci 2006; 114 Department of Pediatric Dentistry & Dental (Suppl. 1): 3–12 Ó Eur J Oral Sci, 2006 Research Institute, Seoul, Korea; 3UCSF School of Dentistry, Department of Growth and Amelogenesis imperfecta (AI) is a heterogeneous group of inherited defects in dental Development, San Francisco, CA, USA; 4 enamel formation. The malformed enamel can be unusually thin, soft, rough and University of Istanbul, Faculty of Dentistry, stained. The strict definition of AI includes only those cases where enamel defects Department of Pedodontics, apa, Istanbul, Turkey; 5The Forsyth Institute, Harvard-Forsyth occur in the absence of other symptoms. Currently, there are seven candidate genes for Department of Oral Biology, Boston, MA, USA AI: amelogenin, enamelin, ameloblastin, tuftelin, distal-less homeobox 3, enamelysin, and kallikrein 4. To identify sequence variations in AI candidate genes in patients with isolated enamel defects, and to deduce the likely effect of each sequence variation on Jan C.-C. -

Journal of Dental Research
Journal of Dental Research http://jdr.sagepub.com/ Cell Differentiation and Matrix Organization in Engineered Teeth A. Nait Lechguer, M.L. Couble, N. Labert, S. Kuchler-Bopp, L. Keller, H. Magloire, F. Bleicher and H. Lesot J DENT RES 2011 90: 583 originally published online 4 February 2011 DOI: 10.1177/0022034510391796 The online version of this article can be found at: http://jdr.sagepub.com/content/90/5/583 Published by: http://www.sagepublications.com On behalf of: International and American Associations for Dental Research Additional services and information for Journal of Dental Research can be found at: Email Alerts: http://jdr.sagepub.com/cgi/alerts Subscriptions: http://jdr.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Apr 13, 2011 OnlineFirst Version of Record - Feb 4, 2011 What is This? Downloaded from jdr.sagepub.com at Service Commun de la Documentation Université de Strasbourg on September 6, 2013 For personal use only. No other uses without permission. © 2011 International & American Associations for Dental Research RESEARCH REPORTS Biomaterials & Bioengineering A. Nait Lechguer1,2, M.L. Couble3,4, N. Labert3,4, S. Kuchler-Bopp1,2, Cell Differentiation and L. Keller1,2, H. Magloire3,4, F. Bleicher3,4, Matrix Organization in and H. Lesot1,2* Engineered Teeth 1INSERM UMR 977, Faculté de Médecine, 11, rue Humann, F-67085 Strasbourg, France; 2Dental School, University of Strasbourg, Strasbourg, France; 3Université de Lyon, Faculté d’Odontologie, Rue Guillaume Paradin, F-69372 Lyon Cedex 08, France; and 4IGFL, CNRS UMR 5242, Ecole Normale Supérieure, 46 Allée d’Italie, 69364, Lyon Cedex 08, France; *corresponding author, [email protected] J Dent Res 90(5):583-589, 2011 ABSTRACT InTRODuCTIOn Embryonic dental cells were used to check a series of criteria to be achieved for tooth engineering. -

Tooth Enamel and Its Dynamic Protein Matrix
International Journal of Molecular Sciences Review Tooth Enamel and Its Dynamic Protein Matrix Ana Gil-Bona 1,2,* and Felicitas B. Bidlack 1,2,* 1 The Forsyth Institute, Cambridge, MA 02142, USA 2 Department of Developmental Biology, Harvard School of Dental Medicine, Boston, MA 02115, USA * Correspondence: [email protected] (A.G.-B.); [email protected] (F.B.B.) Received: 26 May 2020; Accepted: 20 June 2020; Published: 23 June 2020 Abstract: Tooth enamel is the outer covering of tooth crowns, the hardest material in the mammalian body, yet fracture resistant. The extremely high content of 95 wt% calcium phosphate in healthy adult teeth is achieved through mineralization of a proteinaceous matrix that changes in abundance and composition. Enamel-specific proteins and proteases are known to be critical for proper enamel formation. Recent proteomics analyses revealed many other proteins with their roles in enamel formation yet to be unraveled. Although the exact protein composition of healthy tooth enamel is still unknown, it is apparent that compromised enamel deviates in amount and composition of its organic material. Why these differences affect both the mineralization process before tooth eruption and the properties of erupted teeth will become apparent as proteomics protocols are adjusted to the variability between species, tooth size, sample size and ephemeral organic content of forming teeth. This review summarizes the current knowledge and published proteomics data of healthy and diseased tooth enamel, including advancements in forensic applications and disease models in animals. A summary and discussion of the status quo highlights how recent proteomics findings advance our understating of the complexity and temporal changes of extracellular matrix composition during tooth enamel formation. -

6 Development of the Teeth: Root and Supporting Structures Nagat M
AVERY Chap.06 27-11-2002 10:09 Pagina 108 108 II Development of the Teeth and Supporting Structures 6 Development of the Teeth: Root and Supporting Structures Nagat M. ElNesr and James K. Avery Chapter Outline Introduction Introduction... 108 Objectives... 108 Root development is initiated through the contributions Root Sheath Development... 109 of the cells originating from the enamel organ, dental Single-Root Formation... 110 papilla, and dental follicle. The cells of the outer enamel Multiple-Root Formation... 111 epithelium contact the inner enamel epithelium at the Root Formation Anomalies... 112 base of the enamel organ, the cervical loop (Figs. 6.1 and Fate of the Epithelial Root Sheath (Hertwig's Sheath)... 113 6.2A). Later, with crown completion, the cells of the cer- Dental Follicle... 114 vical loop continue to grow away from the crown and Development of (Intermediate) Cementum... 116 become root sheath cells (Figs. 6.2B and 6.3). The inner Cellular and Acellular Cementum... 116 root sheath cells cause root formation by inducing the Development of the Periodontal Ligament... 117 adjacent cells of the dental papilla to become odonto- Development of the Alveolar Process... 119 blasts, which in turn will form root dentin. The root Summary... 121 sheath will further dictate whether the tooth will have Self-Evaluation Review... 122 single or multiple roots. The remainder of the cells of the dental papilla will then become the cells of the root pulp.The third compo- nent in root formation, the dental follicle, is the tissue that surrounds the enamel organ, the dental papilla, and the root. -

Amelogenesis Imperfecta - Literature Review
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 13, Issue 1 Ver. IX. (Feb. 2014), PP 48-51 www.iosrjournals.org Amelogenesis Imperfecta - Literature Review GemimaaHemagaran1 ,Arvind. M2 1B.D.S.,Saveetha Dental College, Chennai, India 2B.D.S., M.D.S., Dip Oral medicine, Prof. of Oral Medicine, Saveetha Dental College, Chennai, India. Abstract: Amelogenesis Imperfecta (AI) is a group of inherited disorder of dental enamel formation in the absence of systemic manifestations. AI is also known as Hereditary enamel dysplasia, Hereditary brown enamel, Hereditary brown opalescent teeth. Since the mesodermal components of the teeth is normal, this defect is entirely ectodermal in origin. Variants of AI generally are classified as hypoplastic, hypocalcified, or hypomineralised types based on the primary enamel defect. The affected teeth may be discoloured, sensitive or prone to disintegration, leading to loss of occlusal vertical dimensions and very poor aesthetics. They exists in isolation or associated with other abnormalities in syndromes. It may show autosomal dominant, autosomal recessive, sex-linked and sporadic inheritance patterns. Mutations in the amelogenin, enamelin, and kallikrein-4 genes have been demonstrated to different types of AI. Keywords: AmelogenesisImperfecta, Hypoplastic, Hypocalcific, Hypomineralised. I. Introduction: Amelogenesis imperfecta (AI) is a term for a clinically and genetically heterogeneous group of conditions that affect the dental enamel, occasionally in conjunction with other dental, oral and extraoral tissues.[1] Dental enamel formation is divided into secretory, transition, and maturation stages. During the secretory stage, enamel crystals grow primarily in length. During the maturation stage, mineral is deposited exclusively on the sides of the crystallites, which grow in width and thickness to coalesce with adjacent crystals.[2]The main structural proteins in forming enamel are amelogenin, ameloblastin, and enamelin. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Specialized Stem Cell Niche Enables Repetitive Renewal of Alligator Teeth
Specialized stem cell niche enables repetitive renewal PNAS PLUS of alligator teeth Ping Wua, Xiaoshan Wua,b, Ting-Xin Jianga, Ruth M. Elseyc, Bradley L. Templed, Stephen J. Diverse, Travis C. Glennd, Kuo Yuanf, Min-Huey Cheng,h, Randall B. Widelitza, and Cheng-Ming Chuonga,h,i,1 aDepartment of Pathology, University of Southern California, Los Angeles, CA 90033; bDepartment of Oral and Maxillofacial Surgery, Xiangya Hospital, Central South University, Hunan 410008, China; cLouisiana Department of Wildlife and Fisheries, Rockefeller Wildlife Refuge, Grand Chenier, LA 70643; dEnvironmental Health Science and eDepartment of Small Animal Medicine and Surgery, University of Georgia, Athens, GA 30602; fDepartment of Dentistry and iResearch Center for Wound Repair and Regeneration, National Cheng Kung University, Tainan City 70101, Taiwan; and gSchool of Dentistry and hResearch Center for Developmental Biology and Regenerative Medicine, National Taiwan University, Taipei 10617, Taiwan Edited by Edward M. De Robertis, Howard Hughes Medical Institute/University of California, Los Angeles, CA, and accepted by the Editorial Board March 28, 2013 (received for review July 31, 2012) Reptiles and fish have robust regenerative powers for tooth renewal. replaced from the dental lamina connected to the lingual side of However, extant mammals can either renew their teeth one time the deciduous tooth (15). Human teeth are only replaced one time; (diphyodont dentition) or not at all (monophyodont dentition). however, a remnant of the dental lamina still exists (16) and may Humans replace their milk teeth with permanent teeth and then become activated later in life to form odontogenic tumors (17). lose their ability for tooth renewal. -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Amelogenic Transcriptome Profiling in Ameloblast-Like Cells Derived From
www.nature.com/scientificreports OPEN Amelogenic transcriptome profling in ameloblast-like cells derived from adult gingival epithelial cells Received: 23 May 2018 Sun-Yi Hyun1, Seyoung Mun1,2, Kyung-Jung Kang1, Jong-Chan Lim1, Shin-Young Kim1, Accepted: 29 January 2019 Kyudong Han1,2 & Young-Joo Jang 1 Published: xx xx xxxx Dental enamel is the highly mineralized tissue covering the tooth surface and is formed by ameloblasts. Ameloblasts have been known to be impossible to detect in adult tooth because they are shed by apoptosis during enamel maturation and tooth eruption. Owing to these, little was known about appropriate cell surface markers to isolate ameloblast-like cells in tissues. To overcome these problems, epithelial cells were selectively cultivated from the gingival tissues and used as a stem cell source for ameloblastic diferentiation. When gingival epithelial cells were treated with a specifed concentration of BMP2, BMP4, and TGFβ-1, the expression of ameloblast-specifc markers was increased, and both the MAPK and Smad signaling pathways were activated. Gingival epithelial cells diferentiated into ameloblast-like cells through epithelial-mesenchymal transition. By RNA-Seq analysis, we reported 20 ameloblast-specifc genes associated with cell surface, cell adhesion, and extracellular matrix function. These cell surface markers might be useful for the detection and isolation of ameloblast-like cells from dental tissues. Dentin, dental pulp, periodontal ligament, and dental enamel are developed by reciprocal interactions between dental epithelium and ectomesenchyme. Neural crest cell-derived ectomesenchyme diferentiates into odonto- blasts, periodontal ligament progenitors, cementoblasts, as well as various fibroblasts. On the other hand, enamel-forming ameloblasts diferentiate from epithelial cells originating from oral ectoderm.